Neuronal Wiskott-Aldrich syndrome protein (N-WASP) is critical for formation of α-smooth muscle actin filaments during myofibroblast differentiation
- PMID: 22886502
- PMCID: PMC3469635
- DOI: 10.1152/ajplung.00390.2011
Neuronal Wiskott-Aldrich syndrome protein (N-WASP) is critical for formation of α-smooth muscle actin filaments during myofibroblast differentiation
Abstract
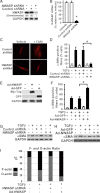
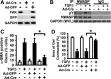
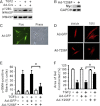
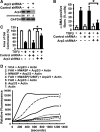
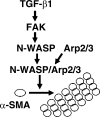
Myofibroblasts are implicated in pathological stromal responses associated with lung fibrosis. One prominent phenotypic marker of fully differentiated myofibroblasts is the polymerized, thick cytoplasmic filaments containing newly synthesized α-smooth muscle actin (α-SMA). These α-SMA-containing cytoplasmic filaments are important for myofibroblast contractility during tissue remodeling. However, the molecular mechanisms regulating the formation and maturation of α-SMA-containing filaments have not been defined. This study demonstrates a critical role for neuronal Wiskott-Aldrich syndrome protein (N-WASP) in regulating the formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and in myofibroblast contractility. Focal adhesion kinase (FAK) is activated by transforming growth factor-β1 (TGF-β1) and is required for phosphorylation of tyrosine residue 256 (Y256) of N-WASP. Phosphorylation of Y256 of N-WASP is essential for TGF-β1-induced formation of α-SMA-containing cytoplasmic filaments in primary human lung fibroblasts. In addition, we demonstrate that actin-related protein (Arp) 2/3 complex is downstream of N-WASP and mediates the maturation of α-SMA-containing cytoplasmic filaments. Together, this study supports a critical role of N-WASP in integrating FAK and Arp2/3 signaling to mediate formation of α-SMA-containing cytoplasmic filaments during myofibroblast differentiation and maturation.
Figures

References
-
- Arora PD, McCulloch CA. Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol 159: 161–175, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

