Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer
- PMID: 22887343
- PMCID: PMC4086804
- DOI: 10.1002/pros.22573
Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer
Abstract
Purpose: This study was conducted to investigate the effect of Se supplementation on prostate cancer incidence in men at high risk for prostate cancer.
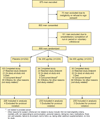
Methods: A Phase 3 randomized, double-blind, placebo-controlled clinical trial was conducted in 699 men at high risk for prostate cancer (prostate specific antigen (PSA) >4 ng/ml and/or suspicious digital rectal examination and/or PSA velocity >0.75 ng/ml/year), but with a negative prostate biopsy. Participants were randomized to receive daily oral placebo (N = 232), 200 µg selenium (N = 234), or 400 µg selenium (N = 233) as selenized yeast. They were followed every 6 months for up to 5 years. The time to diagnosis of prostate cancer was compared between treatment groups using the Cox proportional hazards model.
Result: Compared to placebo, the hazard ratios [95% confidence intervals] for risk of developing prostate cancer in the selenium 200 µg/day or the selenium 400 µg/day group were 0.94 [0.52, 1.7] and 0.90 [0.48, 1.7], respectively. PSA velocity in the selenium arms was not significantly different from that observed in the placebo group (P = 0.18 and P = 0.17, respectively).
Conclusion: Selenium supplementation appeared to have no effect on the incidence of prostate cancer in men at high risk. In conjunction with results of other studies, these data indicate that selenium supplementation may not have a role in prostate cancer chemoprevention.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- ACS, Cancer Facts & Figures. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012.
-
- Clark LC, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276(24):1957–1963. - PubMed
-
- Duffield-Lillico AJ, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11(7):630–639. - PubMed
-
- Stratton MS, et al. Selenium and prevention of prostate cancer in high-risk men: the Negative Biopsy Study. Anticancer Drugs. 2003;14(8):589–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

