Ozone exposure during the early postnatal period alters the timing and pattern of alveolar growth and development in nonhuman primates
- PMID: 22887719
- PMCID: PMC5462601
- DOI: 10.1002/ar.22545
Ozone exposure during the early postnatal period alters the timing and pattern of alveolar growth and development in nonhuman primates
Abstract
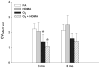
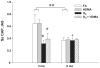
Exposure to oxidant air pollutants in early childhood, with ozone as the key oxidant, has been linked to significant decrements in pulmonary function in young adults and exacerbation of airway remodeling in asthma. Development of lung parenchyma in rhesus monkeys is rapid during the first 2 years of life (comparable to the first 6 years in humans). Our hypothesis is that ozone inhalation during infancy alters alveolar morphogenesis. We exposed infant rhesus monkeys biweekly to 5, 8 hr/day, cycles of 0.5 ppm ozone with or without house dust mite allergen from 1 to 3 or 1 to 6 months of age. Monkeys were necropsied at 3 and 6 months of age. A morphometric approach was used to quantify changes in alveolar volume and number, the distribution of alveolar size, and capillary surface density per alveolar septa. Quantitative real time PCR was used to measure the relative difference in gene expression over time. Monkeys exposed to ozone alone or ozone combined with allergen had statistically larger alveoli that were less in number at 3 months of age. Alveolar capillary surface density was also decreased in the ozone exposed groups at 3 months of age. At 6 months of age, the alveolar number was similar between treatment groups and was associated with a significant rise in alveolar number from 3 to 6 months of age in the ozone exposed groups. This increase in alveolar number was not associated with any significant increase in microvascular growth as measured by morphometry or changes in angiogenic gene expression. Inhalation of ozone during infancy alters the appearance and timing of alveolar growth and maturation. Understanding the mechanism involved with this altered alveolar growth may provide insight into the parenchymal injury and repair process that is involved with chronic lung diseases such as severe asthma and COPD.
Copyright © 2012 Wiley Periodicals, Inc.
Figures






References
-
- Burri PH. Structural aspects of postnatal lung development-alveolar formation and growth. Biol Neonate. 2006;89:313–322. - PubMed
-
- Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585. - PubMed
-
- Delfino RJ, Coate BD, Zeiger RS, Seltzer JM, Street DH, Koutrakis P. Daily asthma severity in relation to personal ozone exposure and outdoor fungal spores. Am J Respir Crit Care Med. 1996;154(Pt 1):633–641. - PubMed
-
- Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, Gaston BM, Bleecker ER, Moore WC. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–389. - PMC - PubMed
-
- Golub MS, Gershwin ME, Hurley LS, Saito WY, Hendrickx AG. Studies of marginal zinc deprivation in rhesus monkeys. IV. Growth of infants in the first year. Am J Clin Nutr. 1984;40:1192–1202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

