Centralspindlin and chromosomal passenger complex behavior during normal and Rappaport furrow specification in echinoderm embryos
- PMID: 22887753
- PMCID: PMC3926696
- DOI: 10.1002/cm.21061
Centralspindlin and chromosomal passenger complex behavior during normal and Rappaport furrow specification in echinoderm embryos
Abstract
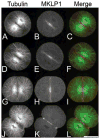
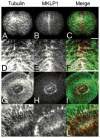
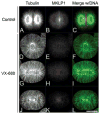
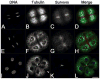
The chromosomal passenger (CPC) and Centralspindlin complexes are essential for organizing the anaphase central spindle and providing cues that position the cytokinetic furrow between daughter nuclei. However, echinoderm zygotes are also capable of forming "Rappaport furrows" between asters positioned back-to-back without intervening chromosomes. To understand how these complexes contribute to normal and Rappaport furrow formation, we studied the localization patterns of Survivin and mitotic-kinesin-like-protein1 (MKLP1), members respectively of the CPC and the Centralspindlin complex, and the effect of CPC inhibition on cleavage in mono- and binucleate echinoderm zygotes. In zygotes, Survivin initially localized to metaphase chromosomes, upon anaphase onset relocalized to the central spindle and then, together with MKLP1 spread towards the equatorial cortex in an Aurora-dependent manner. Inhibition of Aurora kinase activity resulted in disruption of central spindle organization and furrow regression, although astral microtubule elongation and furrow initiation were normal. In binucleate cells containing two parallel spindles MKLP1 and Survivin localized to the plane of the former metaphase plate, but were not observed in the secondary cleavage plane formed between unrelated spindle poles, except when chromosomes were abnormally present there. However, the secondary furrow was sensitive to Aurora inhibition, indicating that Aurora kinase may still contribute to furrow ingression without chromosomes nearby. Our results provide insights that reconcile classic micromanipulation studies with current molecular understanding of furrow specification in animal cells.
Copyright © 2012 Wiley Periodicals, Inc.
Figures




Similar articles
-
The chromosomal passenger complex and centralspindlin independently contribute to contractile ring assembly.J Cell Biol. 2011 Apr 4;193(1):155-69. doi: 10.1083/jcb.201008138. J Cell Biol. 2011. PMID: 21464231 Free PMC article.
-
Centralspindlin in Rappaport's cleavage signaling.Semin Cell Dev Biol. 2016 May;53:45-56. doi: 10.1016/j.semcdb.2016.03.006. Epub 2016 Mar 7. Semin Cell Dev Biol. 2016. PMID: 26964770 Review.
-
Chromosome segregation regulation in human zygotes: altered mitotic histone phosphorylation dynamics underlying centromeric targeting of the chromosomal passenger complex.Hum Reprod. 2015 Oct;30(10):2275-91. doi: 10.1093/humrep/dev186. Epub 2015 Jul 29. Hum Reprod. 2015. PMID: 26223676
-
RhoGEF and positioning of rappaport-like furrows in the early Drosophila embryo.Curr Biol. 2012 Nov 6;22(21):2037-41. doi: 10.1016/j.cub.2012.08.046. Epub 2012 Sep 27. Curr Biol. 2012. PMID: 23022066 Free PMC article.
-
Role of chromosomal passenger complex in chromosome segregation and cytokinesis.Cell Struct Funct. 2001 Dec;26(6):653-7. doi: 10.1247/csf.26.653. Cell Struct Funct. 2001. PMID: 11942622 Review.
Cited by
-
Spindle-to-cortex communication in cleaving, polyspermic Xenopus eggs.Mol Biol Cell. 2015 Oct 15;26(20):3628-40. doi: 10.1091/mbc.E15-04-0233. Epub 2015 Aug 26. Mol Biol Cell. 2015. PMID: 26310438 Free PMC article.
-
Centralspindlin: at the heart of cytokinesis.Cytoskeleton (Hoboken). 2012 Nov;69(11):882-92. doi: 10.1002/cm.21065. Epub 2012 Sep 21. Cytoskeleton (Hoboken). 2012. PMID: 22927365 Free PMC article. Review.
-
Polar relaxation by dynein-mediated removal of cortical myosin II.J Cell Biol. 2020 Aug 3;219(8):e201903080. doi: 10.1083/jcb.201903080. J Cell Biol. 2020. PMID: 32497213 Free PMC article.
-
Furrow constriction in animal cell cytokinesis.Biophys J. 2014 Jan 7;106(1):114-23. doi: 10.1016/j.bpj.2013.11.014. Biophys J. 2014. PMID: 24411243 Free PMC article.
-
Disassembly of Actin and Keratin Networks by Aurora B Kinase at the Midplane of Cleaving Xenopus laevis Eggs.Curr Biol. 2019 Jun 17;29(12):1999-2008.e4. doi: 10.1016/j.cub.2019.05.016. Epub 2019 Jun 6. Curr Biol. 2019. PMID: 31178324 Free PMC article.
References
-
- Abe Y, Okumura E, Hosoya T, Hirota T, Kishimoto T. A single starfish Aurora kinase performs the combined functions of Aurora-A and Aurora-B in human cells. J Cell Sci. 2010;123(Pt 22):3978–3988. - PubMed
-
- Baruni JK, Munro EM, von Dassow G. Cytokinetic furrowing in toroidal, binucleate and anucleate cells in C. elegans embryos. J Cell Sci. 2008;121(Pt 3):306–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

