Peripheral leptin regulates bone formation
- PMID: 22887758
- PMCID: PMC3527690
- DOI: 10.1002/jbmr.1734
Peripheral leptin regulates bone formation
Abstract
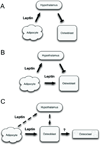
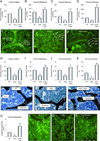
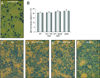
Substantial evidence does not support the prevailing view that leptin, acting through a hypothalamic relay, decreases bone accrual by inhibiting bone formation. To clarify the mechanisms underlying regulation of bone architecture by leptin, we evaluated bone growth and turnover in wild-type (WT) mice, leptin receptor-deficient db/db mice, leptin-deficient ob/ob mice, and ob/ob mice treated with leptin. We also performed hypothalamic leptin gene therapy to determine the effect of elevated hypothalamic leptin levels on osteoblasts. Finally, to determine the effects of loss of peripheral leptin signaling on bone formation and energy metabolism, we used bone marrow (BM) from WT or db/db donor mice to reconstitute the hematopoietic and mesenchymal stem cell compartments in lethally irradiated WT recipient mice. Decreases in bone growth, osteoblast-lined bone perimeter and bone formation rate were observed in ob/ob mice and greatly increased in ob/ob mice following subcutaneous administration of leptin. Similarly, hypothalamic leptin gene therapy increased osteoblast-lined bone perimeter in ob/ob mice. In spite of normal osteoclast-lined bone perimeter, db/db mice exhibited a mild but generalized osteopetrotic-like (calcified cartilage encased by bone) skeletal phenotype and greatly reduced serum markers of bone turnover. Tracking studies and histology revealed quantitative replacement of BM cells following BM transplantation. WT mice engrafted with db/db BM did not differ in energy homeostasis from untreated WT mice or WT mice engrafted with WT BM. Bone formation in WT mice engrafted with WT BM did not differ from WT mice, whereas bone formation in WT mice engrafted with db/db cells did not differ from the low rates observed in untreated db/db mice. In summary, our results indicate that leptin, acting primarily through peripheral pathways, increases osteoblast number and activity.
Copyright © 2013 American Society for Bone and Mineral Research.
Conflict of interest statement
Figures

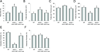


Comment in
-
What are the effects of leptin on bone and where are they exerted?J Bone Miner Res. 2013 Jan;28(1):18-21. doi: 10.1002/jbmr.1812. J Bone Miner Res. 2013. PMID: 23188700 No abstract available.
References
-
- Wong IP, Zengin A, Herzog H, Baldock PA. Central regulation of bone mass. Semin Cell Dev Biol. 2008;19(5):452–458. - PubMed
-
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. - PubMed
-
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317. - PubMed
-
- Graham S, Hammond-Jones D, Gamie Z, Polyzois I, Tsiridis E. The effect of beta-blockers on bone metabolism as potential drugs under investigation for osteoporosis and fracture healing. Expert Opin Investig Drugs. 2008;17(9):1281–1299. - PubMed
-
- Trivedi R, Goswami R, Chattopadhyay N. Investigational anabolic therapies for osteoporosis. Expert Opin Investig Drugs. 2010;19(8):995–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

