SACY-1 DEAD-Box helicase links the somatic control of oocyte meiotic maturation to the sperm-to-oocyte switch and gamete maintenance in Caenorhabditis elegans
- PMID: 22887816
- PMCID: PMC3522166
- DOI: 10.1534/genetics.112.143271
SACY-1 DEAD-Box helicase links the somatic control of oocyte meiotic maturation to the sperm-to-oocyte switch and gamete maintenance in Caenorhabditis elegans
Abstract
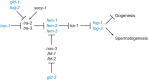
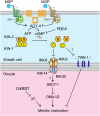
In sexually reproducing animals, oocytes arrest at diplotene or diakinesis and resume meiosis (meiotic maturation) in response to hormones. In Caenorhabditis elegans, major sperm protein triggers meiotic resumption through a mechanism involving somatic Gα(s)-adenylate cyclase signaling and soma-to-germline gap-junctional communication. Using genetic mosaic analysis, we show that the major effector of Gα(s)-adenylate cyclase signaling, protein kinase A (PKA), is required in gonadal sheath cells for oocyte meiotic maturation and dispensable in the germ line. This result rules out a model in which cyclic nucleotides must transit through sheath-oocyte gap junctions to activate PKA in the germ line, as proposed in vertebrate systems. We conducted a genetic screen to identify regulators of oocyte meiotic maturation functioning downstream of Gα(s)-adenylate cyclase-PKA signaling. We molecularly identified 10 regulatory loci, which include essential and nonessential factors. sacy-1, which encodes a highly conserved DEAD-box helicase, is an essential germline factor that negatively regulates meiotic maturation. SACY-1 is a multifunctional protein that establishes a mechanistic link connecting the somatic control of meiotic maturation to germline sex determination and gamete maintenance. Modulatory factors include multiple subunits of a CoREST-like complex and the TWK-1 two-pore potassium channel. These factors are not absolutely required for meiotic maturation or its negative regulation in the absence of sperm, but function cumulatively to enable somatic control of meiotic maturation. This work provides insights into the genetic control of meiotic maturation signaling in C. elegans, and the conserved factors identified here might inform analysis in other systems through either homology or analogy.
Figures



Similar articles
-
Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans.Development. 2009 Jul;136(13):2211-21. doi: 10.1242/dev.034595. Development. 2009. PMID: 19502483 Free PMC article.
-
Control of oocyte growth and meiotic maturation in Caenorhabditis elegans.Adv Exp Med Biol. 2013;757:277-320. doi: 10.1007/978-1-4614-4015-4_10. Adv Exp Med Biol. 2013. PMID: 22872481 Free PMC article. Review.
-
Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans.Curr Biol. 2008 May 20;18(10):705-714. doi: 10.1016/j.cub.2008.04.043. Epub 2008 May 8. Curr Biol. 2008. PMID: 18472420 Free PMC article.
-
Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans.Curr Biol. 2006 Jul 11;16(13):1257-68. doi: 10.1016/j.cub.2006.05.020. Curr Biol. 2006. PMID: 16824915
-
Control of oocyte meiotic maturation in C. elegans.Semin Cell Dev Biol. 2018 Dec;84:90-99. doi: 10.1016/j.semcdb.2017.12.005. Epub 2017 Dec 26. Semin Cell Dev Biol. 2018. PMID: 29242146 Free PMC article. Review.
Cited by
-
DDX41: exploring the roles of a versatile helicase.Biochem Soc Trans. 2024 Feb 28;52(1):395-405. doi: 10.1042/BST20230725. Biochem Soc Trans. 2024. PMID: 38348889 Free PMC article. Review.
-
Two classes of gap junction channels mediate soma-germline interactions essential for germline proliferation and gametogenesis in Caenorhabditis elegans.Genetics. 2014 Nov;198(3):1127-53. doi: 10.1534/genetics.114.168815. Epub 2014 Sep 6. Genetics. 2014. PMID: 25195067 Free PMC article.
-
A histone methylation network regulates transgenerational epigenetic memory in C. elegans.Cell Rep. 2014 Apr 10;7(1):113-26. doi: 10.1016/j.celrep.2014.02.044. Epub 2014 Mar 27. Cell Rep. 2014. PMID: 24685137 Free PMC article.
-
Pharmacological and molecular dynamics analyses of differences in inhibitor binding to human and nematode PDE4: Implications for management of parasitic nematodes.PLoS One. 2019 Mar 27;14(3):e0214554. doi: 10.1371/journal.pone.0214554. eCollection 2019. PLoS One. 2019. PMID: 30917179 Free PMC article.
-
Regulation of Nucleotide Metabolism and Germline Proliferation in Response to Nucleotide Imbalance and Genotoxic Stresses by EndoU Nuclease.Cell Rep. 2020 Feb 11;30(6):1848-1861.e5. doi: 10.1016/j.celrep.2020.01.050. Cell Rep. 2020. PMID: 32049015 Free PMC article.
References
-
- Boxem M., Srinivasan D. G., van den Heuvel S., 1999. The Caenorhabditis elegans gene ncc-1 encodes a cdc2-related kinase required for M phase in meiotic and mitotic cell divisions, but not for S phase. Development 126(10): 2227–2239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

