Bacterial cytokinesis: From Z ring to divisome
- PMID: 22888013
- PMCID: PMC3931253
- DOI: 10.1002/cm.21054
Bacterial cytokinesis: From Z ring to divisome
Abstract
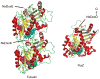
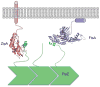
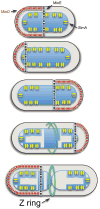
Ancestral homologues of the major eukaryotic cytoskeletal families, tubulin and actin, play critical roles in cytokinesis of bacterial cells. FtsZ is the ancestral homologue of tubulin and assembles into the Z ring that determines the division plane. FtsA, a member of the actin family, is involved in coordinating cell wall synthesis during cytokinesis. FtsA assists in the formation of the Z ring and also has a critical role in recruiting downstream division proteins to the Z ring to generate the divisome that divides the cell. Spatial regulation of cytokinesis occurs at the stage of Z ring assembly and regulation of cell size occurs at this stage or during Z ring maturation.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Aarsman ME, Piette A, Fraipont C, Vinkenvleugel TM, Nguyen-Disteche M, den Blaauwen T. Maturation of the Escherichia coli divisome occurs in two steps. Mol Microbiol. 2005;55:1631–1645. - PubMed
-
- Addinall SG, Lutkenhaus J. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol Microbiol. 1996;22:231–237. - PubMed
-
- Andreu JM, Schaffner-Barbero C, Huecas S, Alonso D, Lopez-Rodriguez ML, Ruiz-Avila LB, Nunez-Ramirez R, Llorca O, Martin-Galiano AJ. The antibacterial cell division inhibitor PC190723 is an FtsZ polymer-stabilizing agent that induces filament assembly and condensation. J Biol Chem. 2010;285:14239–14246. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

