Doripenem dosing recommendations for critically ill patients receiving continuous renal replacement therapy
- PMID: 22888451
- PMCID: PMC3409546
- DOI: 10.5402/2012/782656
Doripenem dosing recommendations for critically ill patients receiving continuous renal replacement therapy
Abstract
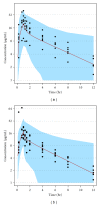
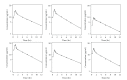
Doripenem dosing regimens for patients receiving continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHDF) were devised based on an established efficacy criterion (free plasma doripenem concentrations above the minimum inhibitory concentration [fT > MIC] of 1 mg/L for ≥35% of the dosing interval) while maintaining exposure below that with the highest studied dose of 1000 mg infused over 1 hour every 8 hours in healthy subjects. Simulations were utilized to assure ≥90% probability of achieving the efficacy criterion with the recommended doripenem regimens. Inflated intersubject variability of 40% (coefficient of variation) was used for pharmacokinetic parameters (representative of clinical variation) and nonrenal clearance was doubled to account for potential changes with acute renal insufficiency. Results indicate that a reduction in doripenem dose will be needed for critically ill patients receiving CVVH or CVVHDF. This work was conducted to fulfill a health authority request and resulted in the addition of dosing recommendations to the Doribax Summary of Product Characteristics.
Figures

References
-
- Jones RN, Huynh HK, Biedenbach DJ, Fritsche TR, Sader HS. Doripenem (S-4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. Journal of Antimicrobial Chemotherapy. 2004;54(1):144–154. - PubMed
-
- Pillar CM, Torres MK, Brown NP, Shah D, Sahm DF. In vitro activity of doripenem, a carbapenem for the treatment of challenging infections caused by gram-negative bacteria, against recent clinical isolates from the United States. Antimicrobial Agents and Chemotherapy. 2008;52(12):4388–4399. - PMC - PubMed
-
- Ortho-McNeil-Janssen Pharmaceuticals, Inc. Doribax Prescribing Information 2007. 2010, http://doribax.com/shared/pi/doribax.pdf.
-
- European Medicines Authority. Summary of product characteristics: Doribax. 2011, http://www.emea.europa.eu/humandocs/Humans/EPAR/doribax/doribax.htm.
-
- Cirillo I, Vaccaro N, Turner K, Solanki B, Natarajan J, Redman R. Pharmacokinetics, safety, and tolerability of doripenem after 0.5-, 1-, and 4-hour infusions in healthy volunteers. Journal of Clinical Pharmacology. 2009;49(7):798–806. - PubMed
LinkOut - more resources
Full Text Sources
