An integrated approach to identify normal tissue expression of targets for antibody-drug conjugates: case study of TENB2
- PMID: 22889168
- PMCID: PMC3572570
- DOI: 10.1111/j.1476-5381.2012.02138.x
An integrated approach to identify normal tissue expression of targets for antibody-drug conjugates: case study of TENB2
Abstract
Background and purpose: The success of antibody-drug conjugates (ADCs) depends on the therapeutic window rendered by the differential expression between normal and pathological tissues. The ability to identify and visualize target expression in normal tissues could reveal causes for target-mediated clearance observed in pharmacokinetic characterization. TENB2 is a prostate cancer target associated with the progression of poorly differentiated and androgen-independent tumour types, and ADCs specific for TENB2 are candidate therapeutics. The objective of this study was to locate antigen expression of TENB2 in normal tissues, thereby elucidating the underlying causes of target-mediated clearance.
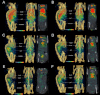
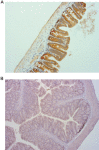
Experimental approach: A series of pharmacokinetics, tissue distribution and mass balance studies were conducted in mice using a radiolabelled anti-TENB2 ADC. These data were complemented by non-invasive single photon emission computed tomography - X-ray computed tomography imaging and immunohistochemistry.
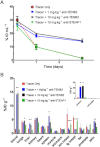
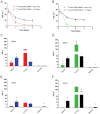
Key results: The intestines were identified as a saturable and specific antigen sink that contributes, at least in part, to the rapid target-mediated clearance of the anti-TENB2 antibody and its drug conjugate in rodents. As a proof of concept, we also demonstrated the selective disposition of the ADC in a tumoural environment in vivo using the LuCaP 77 transplant mouse model. High tumour uptake was observed despite the presence of the antigen sink, and antigen specificity was confirmed by antigen blockade.
Conclusions and implications: Our findings provide the anatomical location and biological interpretation of target-mediated clearance of anti-TENB2 antibodies and corresponding drug conjugates. Further investigations may be beneficial in addressing the relative contributions to ADC disposition from antigen expression in both normal and pathological tissues.
© 2012 Genentech, Inc.. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures




Similar articles
-
Differential effects of predosing on tumor and tissue uptake of an 111In-labeled anti-TENB2 antibody-drug conjugate.J Nucl Med. 2012 Sep;53(9):1454-61. doi: 10.2967/jnumed.112.103168. Epub 2012 Aug 7. J Nucl Med. 2012. PMID: 22872740
-
Impact of drug conjugation on pharmacokinetics and tissue distribution of anti-STEAP1 antibody-drug conjugates in rats.Bioconjug Chem. 2011 Oct 19;22(10):1994-2004. doi: 10.1021/bc200212a. Epub 2011 Oct 3. Bioconjug Chem. 2011. PMID: 21913715
-
ImmunoPET helps predicting the efficacy of antibody-drug conjugates targeting TENB2 and STEAP1.Oncotarget. 2016 May 3;7(18):25103-12. doi: 10.18632/oncotarget.8390. Oncotarget. 2016. PMID: 27029064 Free PMC article.
-
Pharmacokinetic Considerations for Antibody-Drug Conjugates against Cancer.Pharm Res. 2017 Dec;34(12):2579-2595. doi: 10.1007/s11095-017-2259-3. Epub 2017 Sep 18. Pharm Res. 2017. PMID: 28924691 Review.
-
[Progress on pharmacokinetic study of antibody-drug conjugates].Yao Xue Xue Bao. 2015 Oct;50(10):1203-9. Yao Xue Xue Bao. 2015. PMID: 26837163 Review. Chinese.
Cited by
-
Antibody Drug Conjugates: Nonclinical Safety Considerations.AAPS J. 2015 Sep;17(5):1055-64. doi: 10.1208/s12248-015-9790-0. Epub 2015 May 30. AAPS J. 2015. PMID: 26024656 Free PMC article. Review.
-
Production, characterization, and in vivo half-life extension of polymeric IgA molecules in mice.MAbs. 2019 Aug/Sep;11(6):1122-1138. doi: 10.1080/19420862.2019.1622940. Epub 2019 Jun 9. MAbs. 2019. PMID: 31122132 Free PMC article.
-
Payload of T-DM1 binds to cell surface cytoskeleton-associated protein 5 to mediate cytotoxicity of hepatocytes.Oncotarget. 2018 Dec 14;9(98):37200-37215. doi: 10.18632/oncotarget.26461. eCollection 2018 Dec 14. Oncotarget. 2018. PMID: 30647854 Free PMC article.
-
Intravital Microscopy Reveals Unforeseen Biodistribution Within the Liver and Kidney Mechanistically Connected to the Clearance of a Bifunctional Antibody.Drug Metab Dispos. 2023 Mar;51(3):403-412. doi: 10.1124/dmd.122.001049. Epub 2022 Dec 2. Drug Metab Dispos. 2023. PMID: 36460476 Free PMC article.
-
Evolving Strategies for Target Selection for Antibody-Drug Conjugates.Pharm Res. 2015 Nov;32(11):3494-507. doi: 10.1007/s11095-015-1624-3. Epub 2015 Jan 15. Pharm Res. 2015. PMID: 25585957
References
-
- Afar DE, Bhaskar V, Ibsen E, Breinberg D, Henshall SM, Kench JG, et al. Preclinical validation of anti-TMEFF2-auristatin E-conjugated antibodies in the treatment of prostate cancer. Mol Cancer Ther. 2004;3:921–932. - PubMed
-
- Alley SC, Benjamin DR, Jeffrey SC, Okeley NM, Meyer DL, Sanderson RJ, et al. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug Chem. 2008;19:759–765. - PubMed
-
- Alley SC, Zhang X, Okeley NM, Anderson M, Law CL, Senter PD, et al. The pharmacologic basis for antibody-auristatin conjugate activity. J Pharmacol Exp Ther. 2009;330:932–938. - PubMed
-
- Bai RL, Pettit GR, Hamel E. Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem. 1990;265:17141–17149. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

