Substituent effects on desferrithiocin and desferrithiocin analogue iron-clearing and toxicity profiles
- PMID: 22889170
- PMCID: PMC3583384
- DOI: 10.1021/jm300509y
Substituent effects on desferrithiocin and desferrithiocin analogue iron-clearing and toxicity profiles
Abstract
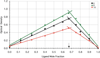
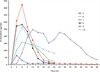
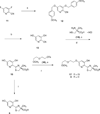
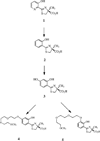
Desferrithiocin (DFT, 1) is a very efficient iron chelator when given orally. However, it is severely nephrotoxic. Structure-activity studies with 1 demonstrated that removal of the aromatic nitrogen to provide desazadesferrithiocin (DADFT, 2) and introduction of either a hydroxyl group or a polyether fragment onto the aromatic ring resulted in orally active iron chelators that were much less toxic than 1. The purpose of the current study was to determine if a comparable reduction in renal toxicity could be achieved by performing the same structural manipulations on 1 itself. Accordingly, three DFT analogues were synthesized. The iron-clearing efficiency and ferrokinetics were evaluated in rats and primates; toxicity assessments were carried out in rodents. The resulting DFT ligands demonstrated a reduction in toxicity that was equivalent to that of the DADFT analogues and presented with excellent iron-clearing properties.
Figures



References
-
- Byers BR, Arceneaux JE. Microbial Iron Transport: Iron Acquisition by Pathogenic Microorganisms. Met. Ions Biol. Syst. 1998;35:37–66. - PubMed
-
- Bergeron RJ. Iron: A Controlling Micronutrient in Proliferative Processes. Trends Biochem. Sci. 1986;11:133–136.
-
- Theil EC, Huynh BH. Ferritin Mineralization: Ferroxidation and Beyond. J. Inorg. Biochem. 1997;67:30.
-
- Ponka P, Beaumont C, Richardson DR. Function and Regulation of Transferrin and Ferritin. Semin. Hematol. 1998;35:35–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

