Cholesterol accumulation increases insulin granule size and impairs membrane trafficking
- PMID: 22889194
- PMCID: PMC3465494
- DOI: 10.1111/j.1600-0854.2012.01407.x
Cholesterol accumulation increases insulin granule size and impairs membrane trafficking
Abstract
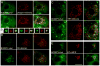
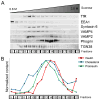
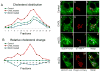
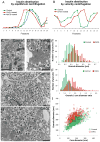
The formation of mature secretory granules is essential for proper storage and regulated release of hormones and neuropeptides. In pancreatic β cells, cholesterol accumulation causes defects in insulin secretion and may participate in the pathogenesis of type 2 diabetes. Using a novel cholesterol analog, we show for the first time that insulin granules are the major sites of intracellular cholesterol accumulation in live β cells. This is distinct from other, non-secretory cell types, in which cholesterol is concentrated in the recycling endosomes and the trans-Golgi network. Excess cholesterol was delivered specifically to insulin granules, which caused granule enlargement and retention of syntaxin 6 and VAMP4 in granule membranes, with concurrent depletion of these proteins from the trans-Golgi network. Clathrin also accumulated in the granules of cholesterol-overloaded cells, consistent with a possible defect in the last stage of granule maturation, during which clathrin-coated vesicles bud from the immature granules. Excess cholesterol also reduced the docking and fusion of insulin granules at the plasma membrane. Together, the data support a model in which cholesterol accumulation in insulin secretory granules impairs the ability of these vesicles to respond to stimuli, and thus reduces insulin secretion.
© 2012 John Wiley & Sons A/S.
Figures
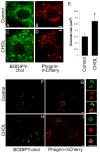

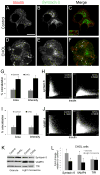

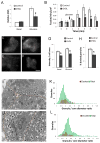
References
-
- Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44(8):863–870. - PubMed
-
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13(3):340–347. - PubMed
-
- Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007;56(9):2328–2338. - PubMed
-
- Fryirs M, Barter PJ, Rye KA. Cholesterol metabolism and pancreatic beta-cell function. Curr Opin Lipidol. 2009;20(3):159–164. - PubMed
-
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438(7068):612–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

