Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide
- PMID: 22889198
- PMCID: PMC3465236
- DOI: 10.1186/1472-6750-12-49
Effect of different freezing rates during cryopreservation of rat mesenchymal stem cells using combinations of hydroxyethyl starch and dimethylsulfoxide
Abstract
Background: Mesenchymal stem cells (MSCs) are increasingly used as therapeutic agents as well as research tools in regenerative medicine. Development of technologies which allow storing and banking of MSC with minimal loss of cell viability, differentiation capacity, and function is required for clinical and research applications. Cryopreservation is the most effective way to preserve cells long term, but it involves potentially cytotoxic compounds and processing steps. Here, we investigate the effect of decreasing dimethyl sulfoxide (DMSO) concentrations in cryosolution by substituting with hydroxyethyl starch (HES) of different molecular weights using different freezing rates. Post-thaw viability, phenotype and osteogenic differentiation capacity of MSCs were analysed.
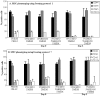
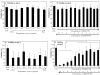
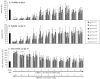
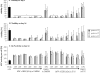
Results: The study confirms that, for rat MSC, cryopreservation effects need to be assessed some time after, rather than immediately after thawing. MSCs cryopreserved with HES maintain their characteristic cell surface marker expression as well as the osteogenic, adipogenic and chondrogenic differentiation potential. HES alone does not provide sufficient cryoprotection for rat MSCs, but provides good cryoprotection in combination with DMSO, permitting the DMSO content to be reduced to 5%. There are indications that such a combination would seem useful not just for the clinical disadvantages of DMSO but also based on a tendency for reduced osteogenic differentiation capacity of rat MSC cryopreserved with high DMSO concentration. HES molecular weight appears to play only a minor role in its capacity to act as a cryopreservation solution for MSC. The use of a 'straight freeze' protocol is no less effective in maintaining post-thaw viability of MSC compared to controlled rate freezing methods.
Conclusion: A 5% DMSO / 5% HES solution cryopreservation solution using a 'straight freeze' approach can be recommended for rat MSC.
Figures
Similar articles
-
Trehalose Cryopreservation of Human Mesenchymal Stem Cells from Cord Tissue.Biopreserv Biobank. 2025 Aug;23(4):374-382. doi: 10.1089/bio.2024.0025. Epub 2024 Dec 26. Biopreserv Biobank. 2025. PMID: 39723442
-
Cryopreservation of mesenchymal stem/stromal cells using a DMSO-free solution is comparable to DMSO-containing cryoprotectants: results of an international multicenter PACT/BEST collaborative study.Cytotherapy. 2024 Dec;26(12):1522-1531. doi: 10.1016/j.jcyt.2024.07.001. Epub 2024 Jul 6. Cytotherapy. 2024. PMID: 39066775
-
Efficient recovery of undifferentiated human embryonic stem cell cryopreserved with hydroxyethyl starch, dimethyl sulphoxide and serum replacement.Cryobiology. 2015 Aug;71(1):151-60. doi: 10.1016/j.cryobiol.2015.01.005. Epub 2015 Jan 29. Cryobiology. 2015. PMID: 25641609
-
Impact of lower concentrations of dimethyl sulfoxide on cryopreservation of autologous hematopoietic stem cells: a systematic review and meta-analysis of controlled clinical studies.Cytotherapy. 2024 May;26(5):482-489. doi: 10.1016/j.jcyt.2024.02.006. Epub 2024 Feb 21. Cytotherapy. 2024. PMID: 38416086
-
Hydroxyethylstarch in cryopreservation - mechanisms, benefits and problems.Transfus Apher Sci. 2012 Apr;46(2):137-47. doi: 10.1016/j.transci.2012.01.007. Epub 2012 Feb 19. Transfus Apher Sci. 2012. PMID: 22349548 Review.
Cited by
-
Cryopreservation of pig spermatozoa using carboxylated poly-L-lysine as cryoprotectant.J Reprod Dev. 2022 Oct 6;68(5):312-317. doi: 10.1262/jrd.2022-058. Epub 2022 Jul 29. J Reprod Dev. 2022. PMID: 35908977 Free PMC article.
-
Maxillofacial-Derived Mesenchymal Stem Cells: Characteristics and Progress in Tissue Regeneration.Stem Cells Int. 2021 Aug 6;2021:5516521. doi: 10.1155/2021/5516521. eCollection 2021. Stem Cells Int. 2021. PMID: 34426741 Free PMC article. Review.
-
The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: a systematic review.J Transl Med. 2019 Nov 29;17(1):397. doi: 10.1186/s12967-019-02136-7. J Transl Med. 2019. PMID: 31783866 Free PMC article.
-
Optimizing cryopreservation conditions for use of fucosylated human mesenchymal stromal cells in anti-inflammatory/immunomodulatory therapeutics.Front Immunol. 2024 Mar 28;15:1385691. doi: 10.3389/fimmu.2024.1385691. eCollection 2024. Front Immunol. 2024. PMID: 38605955 Free PMC article.
-
Harnessing the Secretome of Mesenchymal Stromal Cells for Traumatic Spinal Cord Injury: Multicell Comparison and Assessment of In Vivo Efficacy.Stem Cells Dev. 2020 Nov 15;29(22):1429-1443. doi: 10.1089/scd.2020.0079. Epub 2020 Oct 21. Stem Cells Dev. 2020. PMID: 32962528 Free PMC article.
References
-
- Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001;938:231–233. discussion 233–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

