Metabolic reprogramming and metabolic dependency in T cells
- PMID: 22889212
- PMCID: PMC3422760
- DOI: 10.1111/j.1600-065X.2012.01155.x
Metabolic reprogramming and metabolic dependency in T cells
Abstract
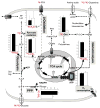
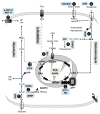
Upon activation, quiescent naive T cells undergo a growth phase followed by massive clonal expansion and differentiation that are essential for appropriate immune defense and regulation. Accumulation of cell biomass during the initial growth and rapid proliferation during the expansion phase is associated with dramatically increased bioenergetic and biosynthetic demands. This not only requires a metabolic rewiring during the transition between resting and activation but also 'addicts' active T cells to certain metabolic pathways in ways that naive and memory T cells are not. We consider such addiction in terms of the biological effects of deprivation of metabolic substrates or inhibition of specific pathways in T cells. In this review, we illustrate the relevant metabolic pathways revealed by recent metabolic flux analysis and discuss the consequences of metabolic intervention on specific metabolic pathways in T lymphocytes.
© 2012 John Wiley & Sons A/S.
Figures
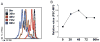

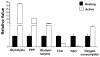
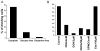
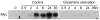
References
-
- Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393–400. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

