Paracrine release from nonviral engineered adipose-derived stem cells promotes endothelial cell survival and migration in vitro
- PMID: 22889246
- PMCID: PMC3549626
- DOI: 10.1089/scd.2012.0201
Paracrine release from nonviral engineered adipose-derived stem cells promotes endothelial cell survival and migration in vitro
Abstract
Stem cells hold great potential for therapeutic angiogenesis due to their ability to directly contribute to new vessel formation or secrete paracrine signals. Adipose-derived stem cells (ADSCs) are a particularly attractive autologous cell source for therapeutic angiogenesis due to their ease of isolation and relative abundance. Gene therapy may be used to further enhance the therapeutic efficacy of ADSCs by overexpressing desired therapeutic factors. Here, we developed vascular endothelial growth factor (VEGF)-overexpressing ADSCs utilizing poly(β-amino esters) (PBAEs), a hydrolytically biodegradable polymer, and examined the effects of paracrine release from nonviral modified ADSCs on the angiogenic potential of human umbilical vein endothelial cells (HUVECs) in vitro. PBAE polymeric vectors delivered DNA into ADSCs with high efficiency and low cytotoxicity, leading to an over 3-fold increase in VEGF production by ADSCs compared with Lipofectamine 2000. Paracrine release from PBAE/VEGF-transfected ADSCs enhanced HUVEC viability and decreased HUVEC apoptosis under hypoxia. Further, paracrine release from PBAE/VEGF-transfected ADSCs significantly enhanced HUVEC migration and tube formation, two critical cellular processes for effective angiogenesis. Our results demonstrate that genetically engineered ADSCs using biodegradable polymeric nanoparticles may provide a promising autologous cell source for therapeutic angiogenesis in treating cardiovascular diseases.
Figures

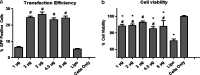





Similar articles
-
Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing.Mol Ther. 2013 Feb;21(2):445-55. doi: 10.1038/mt.2012.234. Epub 2012 Nov 20. Mol Ther. 2013. PMID: 23164936 Free PMC article.
-
Conditioned Medium from Human Umbilical Vein Endothelial Cells Promotes Proliferation, Migration, Invasion and Angiogenesis of Adipose Derived Stem Cells.Curr Med Sci. 2018 Feb;38(1):124-130. doi: 10.1007/s11596-018-1855-8. Epub 2018 Mar 15. Curr Med Sci. 2018. PMID: 30074161
-
Hypoxia-regulated miR-103-3p/FGF2 axis in adipose-derived stem cells promotes angiogenesis by vascular endothelial cells during ischemic tissue repair.Int J Cardiol. 2025 Apr 15;425:133004. doi: 10.1016/j.ijcard.2025.133004. Epub 2025 Jan 24. Int J Cardiol. 2025. PMID: 39864666
-
Improved fat transplantation survival by using the conditioned medium of vascular endothelial growth factor transfected human adipose-derived stem cells.Kaohsiung J Med Sci. 2017 Aug;33(8):379-384. doi: 10.1016/j.kjms.2017.05.009. Epub 2017 May 31. Kaohsiung J Med Sci. 2017. PMID: 28811006 Free PMC article.
-
The miR-590-3p/VEGFA axis modulates secretion of VEGFA from adipose-derived stem cells, which acts as a paracrine regulator of human dermal microvascular endothelial cell angiogenesis.Hum Cell. 2020 Jul;33(3):479-489. doi: 10.1007/s13577-019-00315-8. Epub 2020 Apr 10. Hum Cell. 2020. PMID: 32277427
Cited by
-
Autophagy regulates the therapeutic potential of adipose-derived stem cells in LPS-induced pulmonary microvascular barrier damage.Cell Death Dis. 2019 Oct 23;10(11):804. doi: 10.1038/s41419-019-2037-8. Cell Death Dis. 2019. PMID: 31645547 Free PMC article.
-
Microvesicles (MIVs) secreted from adipose-derived stem cells (ADSCs) contain multiple microRNAs and promote the migration and invasion of endothelial cells.Genes Dis. 2019 Apr 25;7(2):225-234. doi: 10.1016/j.gendis.2019.04.005. eCollection 2020 Jun. Genes Dis. 2019. PMID: 32215292 Free PMC article.
-
Nucleic acid delivery to mesenchymal stem cells: a review of nonviral methods and applications.J Biol Eng. 2019 Jan 18;13:7. doi: 10.1186/s13036-019-0140-0. eCollection 2019. J Biol Eng. 2019. PMID: 30675180 Free PMC article. Review.
-
Harnessing the secretome of adipose-derived stem cells in the treatment of ischemic heart diseases.Stem Cell Res Ther. 2019 Jun 27;10(1):196. doi: 10.1186/s13287-019-1289-7. Stem Cell Res Ther. 2019. PMID: 31248452 Free PMC article. Review.
-
Adipose-derived stem cell-derived microvesicle-released miR-210 promoted proliferation, migration and invasion of endothelial cells by regulating RUNX3.Cell Cycle. 2018;17(8):1026-1033. doi: 10.1080/15384101.2018.1480207. Epub 2018 Jul 5. Cell Cycle. 2018. PMID: 29912616 Free PMC article.
References
-
- Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. - PubMed
-
- Potente M. Gerhardt H. Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. - PubMed
-
- Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part II: cell-based therapies. Circulation. 2004;109:2692–2697. - PubMed
-
- Gruber R. Kandler B. Holzmann P. Vogele-Kadletz M. Losert U. Fischer MB. Watzek G. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11:896–903. - PubMed
-
- Kamihata H. Matsubara H. Nishiue T. Fujiyama S. Tsutsumi Y. Ozono R. Masaki H. Mori Y. Iba O, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

