Expression of early developmental markers predicts the efficiency of embryonic stem cell differentiation into midbrain dopaminergic neurons
- PMID: 22889265
- PMCID: PMC3549628
- DOI: 10.1089/scd.2012.0238
Expression of early developmental markers predicts the efficiency of embryonic stem cell differentiation into midbrain dopaminergic neurons
Abstract
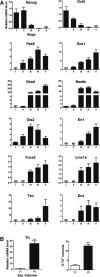
Dopaminergic neurons derived from pluripotent stem cells are among the best investigated products of in vitro stem cell differentiation owing to their potential use for neurorestorative therapy of Parkinson's disease. However, the classical differentiation protocols for both mouse and human pluripotent stem cells generate a limited percentage of dopaminergic neurons and yield a considerable cellular heterogeneity comprising numerous scarcely characterized cell populations. To improve pluripotent stem cell differentiation protocols for midbrain dopaminergic neurons, we established extensive and strictly quantitative gene expression profiles, including markers for pluripotent cells, neural progenitors, non-neural cells, pan-neuronal and glial cells, neurotransmitter phenotypes, midbrain and nonmidbrain populations, floor plate and basal plate populations, as well as for Hedgehog, Fgf, and Wnt signaling pathways. The profiles were applied to discrete stages of in vitro differentiation of mouse embryonic stem cells toward the dopaminergic lineage and after transplantation into the striatum of 6-hydroxy-dopamine-lesioned rats. The comparison of gene expression in vitro with stages in the developing ventral midbrain between embryonic day 11.5 and 13.5 ex vivo revealed dynamic changes in the expression of transcription factors and signaling molecules. Based on these profiles, we propose quantitative gene expression milestones that predict the efficiency of dopaminergic differentiation achieved at the end point of the protocol, already at earlier stages of differentiation.
Figures







References
-
- Lee SH. Lumelsky N. Studer L. Auerbach JM. McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. - PubMed
-
- Barberi T. Klivenyi P. Calingasan NY. Lee H. Kawamata H, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. - PubMed
-
- Kim JH. Auerbach JM. Rodriguez-Gómez JA. Velasco I. Gavin D, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

