Can electromagnetic fields influence the structure and enzymatic digest of proteins? A critical evaluation of microwave-assisted proteomics protocols
- PMID: 22889711
- PMCID: PMC3484400
- DOI: 10.1016/j.jprot.2012.07.043
Can electromagnetic fields influence the structure and enzymatic digest of proteins? A critical evaluation of microwave-assisted proteomics protocols
Abstract
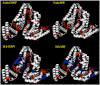
This study reevaluates the putative advantages of microwave-assisted tryptic digests compared to conventionally heated protocols performed at the same temperature. An initial investigation of enzyme stability in a temperature range of 37-80 °C demonstrated that trypsin activity declines sharply at temperatures above 60 °C, regardless if microwave dielectric heating or conventional heating is employed. Tryptic digests of three proteins of different size (bovine serum albumin, cytochrome c and β-casein) were thus performed at 37 °C and 50 °C using both microwave and conventional heating applying accurate internal fiber-optic probe reaction temperature measurements. The impact of the heating method on protein degradation and peptide fragment generation was analyzed by SDS-PAGE and MALDI-TOF-MS. Time-dependent tryptic digestion of the three proteins and subsequent analysis of the corresponding cleavage products by MALDI-TOF provided virtually identical results for both microwave and conventional heating. In addition, the impact of electromagnetic field strength on the tertiary structure of trypsin and BSA was evaluated by molecular mechanics calculations. These simulations revealed that the applied field in a typical laboratory microwave reactor is 3-4 orders of magnitude too low to induce conformational changes in proteins or enzymes.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
On particle ionization/enrichment of multifunctional nanoprobes: washing/separation-free, acceleration and enrichment of microwave-assisted tryptic digestion of proteins via bare TiO2 nanoparticles in ESI-MS and comparing to MALDI-MS.J Mass Spectrom. 2010 Dec;45(12):1402-8. doi: 10.1002/jms.1855. J Mass Spectrom. 2010. PMID: 20967754
-
Advances in nanomaterial-based microwaves and infrared wave-assisted tryptic digestion for ultrafast proteolysis and rapid detection by MALDI-MS.Comb Chem High Throughput Screen. 2014 Jan;17(1):68-79. doi: 10.2174/1386207316666131110211353. Comb Chem High Throughput Screen. 2014. PMID: 24206112 Review.
-
Ultrafast microwave-assisted in-tip digestion of proteins.J Proteome Res. 2009 Sep;8(9):4225-30. doi: 10.1021/pr900188x. J Proteome Res. 2009. PMID: 19639939
-
Effects of incubation temperature and acetonitrile amount on microwave-assisted tryptic digestion of proteins.Anal Biochem. 2019 Mar 15;569:31-38. doi: 10.1016/j.ab.2019.01.009. Epub 2019 Jan 29. Anal Biochem. 2019. PMID: 30707897
-
Evaluating the potential nonthermal microwave effects of microwave-assisted proteolytic reactions.J Proteomics. 2013 Mar 27;80:160-70. doi: 10.1016/j.jprot.2013.01.005. Epub 2013 Jan 24. J Proteomics. 2013. PMID: 23352896
Cited by
-
Skin Fibroblasts from Individuals Self-Diagnosed as Electrosensitive Reveal Two Distinct Subsets with Delayed Nucleoshuttling of the ATM Protein in Common.Int J Mol Sci. 2025 May 16;26(10):4792. doi: 10.3390/ijms26104792. Int J Mol Sci. 2025. PMID: 40429933 Free PMC article.
-
Combined microwave processing and enzymatic proteolysis of bovine whey proteins: the impact on bovine β-lactoglobulin allergenicity.J Food Sci Technol. 2019 Jan;56(1):177-186. doi: 10.1007/s13197-018-3471-9. Epub 2018 Nov 8. J Food Sci Technol. 2019. PMID: 30728559 Free PMC article.
-
Processed Meat Protein and Heat-Stable Peptide Marker Identification Using Microwave-Assisted Tryptic Digestion.Food Technol Biotechnol. 2016 Dec;54(4):482-488. doi: 10.17113/ftb.54.04.16.4540. Food Technol Biotechnol. 2016. PMID: 28115907 Free PMC article.
-
Peptide Markers for Rapid Detection of KPC Carbapenemase by LC-MS/MS.Sci Rep. 2017 May 31;7(1):2531. doi: 10.1038/s41598-017-02749-2. Sci Rep. 2017. PMID: 28566732 Free PMC article.
-
Application of Microwave Irradiation and Heat to Improve Gliadin Detection and Ricin ELISA Throughput with Food Samples.Toxins (Basel). 2015 Jun 11;7(6):2135-44. doi: 10.3390/toxins7062135. Toxins (Basel). 2015. PMID: 26110503 Free PMC article.
References
-
- Lill J.R. RSC Publishing; Cambridge: 2009. Microwave assisted proteomics.
-
- Zhao H. Microwave-assisted enzymatic reactions in aqueous media. In: Polshettiwar V., Varma R.S., editors. Aqueous microwave assisted chemistry. RSC Publishing; Cambridge: 2010. pp. 123–144.
-
- Lill J.R., Ingle E.S., Liu P.S., Pham V., Sandoval W.N. Microwave-assisted proteomics. Mass Spectrom Rev. 2007;26:657–671. - PubMed
-
- Sandoval W.N., Pham V., Ingle E.S., Liu P.S., Lill J.R. Applications of microwave-assisted proteomics in biotechnology. Comb Chem High Throughput Screen. 2007;10:751–765. - PubMed
-
- Sandoval W.N., Pham V.C., Lill J.R. Recent developments in microwave-assisted protein chemistries — can this be integrated into the drug discovery and validation process? Drug Discov Today. 2008;13:1075–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

