Centerline tracking for quantification of reverse structural remodeling of the pulmonary veins following cardiac ablation therapy
- PMID: 22889735
- PMCID: PMC3483868
- DOI: 10.1016/j.acra.2012.06.009
Centerline tracking for quantification of reverse structural remodeling of the pulmonary veins following cardiac ablation therapy
Abstract
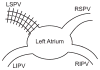
Rationale and objectives: Patients with atrial fibrillation undergo structural remodeling resulting in increased pulmonary vein sizes. Studies have demonstrated that these changes are reversible following successful ablation therapy. To date, analyses of pulmonary vein structure have focused on measurements at the pulmonary vein ostia, and the full extent of reverse remodeling along the length of the pulmonary veins has not yet been fully characterized.
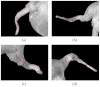
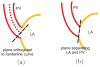
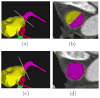
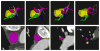
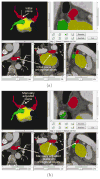
Materials and methods: An automated, three-dimensional method is proposed that quantifies pulmonary vein geometry starting at the ostia and extending several centimeters into the veins. A centerline is tracked along the length of the pulmonary vein, and orthogonal planes are computed along the curve. The method was validated against manual measurements on each of the four pulmonary veins for 10 subjects. The proposed methodology was used to analyze the pulmonary veins in 21 patients undergoing cardiac ablation therapy with preoperative and postoperative computed tomographic scans.
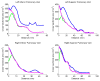
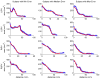
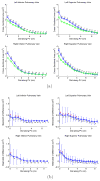
Results: Validation results demonstrated that the automated measurements closely followed the manual measurements, with an overall mean difference of 11.50 mm(2). Significant differences in cross-sectional area at the two time points were observed at all pulmonary vein ostia and extending for 2.0 cm (excluding the 0.5-cm interval) into the left inferior pulmonary vein, 3.5 cm into the left superior pulmonary vein, and 2.0 cm into the right superior pulmonary vein.
Conclusions: Quantitative analysis along the length of the pulmonary veins can be accomplished using centerline tracking and measurements from orthogonal planes along the curve. The patient study demonstrated that reverse structural remodeling following ablation therapy occurs not only at the ostia but for several centimeters extending into the pulmonary veins.
Copyright © 2012 AUR. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
Mayo Clinic and R.A. Robb have a financial interest in technology referenced in this article. Dr. D. Packer in the past 12 months has provided consulting services for Biosense Webster, Inc., CardioFocus, Cardiomedics, Cyberheart, Endosense, Johnson & Johnson Healthcare Systems, Medtronic CryoCath, OrthoMcNeill, Siemens, St. Jude Medical, Siemens AG, and Valencia Technologies. Dr. Packer received no personal compensation for these consulting activities. Dr. Packer receives research funding from the Biosense Webster, Boston Scientific, Endosense, EpiEP, EP Advocate, Medtronic CryoCath LP, Minnesota Partnership for Biotechnology and Medical Genomics/ University of Minnesota, NIH, St. Jude Medical, Siemens AcuNav, and Ther-medical (EP Limited). Royalties from Blackwell Publishing and St. Jude Medical. Mayo Clinic and Drs. D. Packer and R. Robb have a financial interest in mapping technology that may have been used at some of the 10 centers participating in this pilot research. In accordance with the Bayh-Dole Act, this technology has been licensed to St. Jude Medical, and Mayo Clinic and Drs. Packer and Robb have received annual royalties greater than $10,000, the federal threshold for significant financial interest. Mayo Clinic and Dr. R. Robb have a financial interest in Analyze-AVW technology that was used to analyze some of the heart images in this research. In accordance with the Bayh-Dole Act, this technology has been licensed to commercial entities, and both Mayo Clinic and Dr. Robb have received royalties greater than $10,000, the federal threshold for significant financial interest. In addition, Mayo Clinic holds an equity position in the company to which the AVW technology has been licensed.
Figures


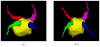
References
-
- Häissaguerre M, Jäis P, Shah D, Takahashi A, Hocini M, Quiniou G, Garrigue S, Mouroux AL, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. - PubMed
-
- Kato R, Lickfett L, Meininger G, Dickfeld T, Wu R, Juang G, Angkeow P, LaCorte J, Bluemke D, Berger R, Halperin H, Calkins H. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation. Circulation. 2003;107:2004–2010. - PubMed
-
- Knackstedt C, Visser L, Plisiene J, Zarse M, Waldmann M, Mischke K, Koch KC, Hoffmann R, Franke A, Hanrath P, Schauerte P. Dilation of the pulmonary veins in atrial fibrillation: A transesophageal echocardiographic evaluation. PACE. 2003;26:1371–1378. - PubMed
-
- Tsao HM, Yu WC, Cheng HC, Wu MH, Tai CT, Lin WS, Ding YA, Chang MS, Chen SA. Pulmonary vein dilation in patients with atrial fibrillation: Detection by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2001;12:809–813. - PubMed
-
- Ueda M, Tada H, Kurosaki K, Itoi K, Koyama K, Naito S, Ito S, Komuro I, Oshima S, Taniguchi K. Pulmonary vein morphology before and after segmental isolation in patients with atrial fibrillation. PACE. 2005;28:944–953. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

