A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds
- PMID: 22889846
- PMCID: PMC3463631
- DOI: 10.1016/j.ajpath.2012.06.036
A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds
Abstract
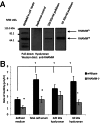
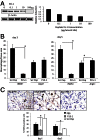
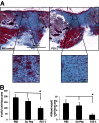
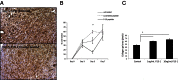
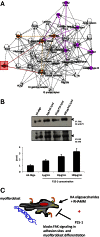
Hyaluronan is activated by fragmentation and controls inflammation and fibroplasia during wound repair and diseases (eg, cancer). Hyaluronan-binding peptides were identified that modify fibrogenesis during skin wound repair. Peptides were selected from 7- to 15mer phage display libraries by panning with hyaluronan-Sepharose beads and assayed for their ability to block fibroblast migration in response to hyaluronan oligosaccharides (10 kDa). A 15mer peptide (P15-1), with homology to receptor for hyaluronan mediated motility (RHAMM) hyaluronan binding sequences, was the most effective inhibitor. P15-1 bound to 10-kDa hyaluronan with an affinity of K(d) = 10(-7) and appeared to specifically mimic RHAMM since it significantly reduced binding of hyaluronan oligosaccharides to recombinant RHAMM but not to recombinant CD44 or TLR2,4, and altered wound repair in wild-type but not RHAMM(-/-) mice. One topical application of P15-1 to full-thickness excisional rat wounds significantly reduced wound macrophage number, fibroblast number, and blood vessel density compared to scrambled, negative control peptides. Wound collagen 1, transforming growth factor β-1, and α-smooth muscle actin were reduced, whereas tenascin C was increased, suggesting that P15-1 promoted a form of scarless healing. Signaling/microarray analyses showed that P15-1 blocks RHAMM-regulated focal adhesion kinase pathways in fibroblasts. These results identify a new class of reagents that attenuate proinflammatory, fibrotic repair by blocking hyaluronan oligosaccharide signaling.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures






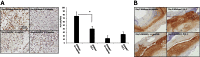
References
-
- Veiseh M., Turley E.A. Hyaluronan metabolism in remodeling extracellular matrix: probes for imaging and therapy of breast cancer. Integr Biol (Camb) 2011;3:304–315. - PubMed
-
- Jiang D., Liang J., Noble P.W. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. - PubMed
-
- Volpi N., Schiller J., Stern R., Soltes L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16:1718–1745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

