Evolution of the eukaryotic protein kinases as dynamic molecular switches
- PMID: 22889904
- PMCID: PMC3415842
- DOI: 10.1098/rstb.2012.0054
Evolution of the eukaryotic protein kinases as dynamic molecular switches
Abstract
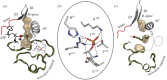
Protein kinases have evolved in eukaryotes to be highly dynamic molecular switches that regulate a plethora of biological processes. Two motifs, a dynamic activation segment and a GHI helical subdomain, distinguish the eukaryotic protein kinases (EPKs) from the more primitive eukaryotic-like kinases. The EPKs are themselves highly regulated, typically by phosphorylation, and this allows them to be rapidly turned on and off. The EPKs have a novel hydrophobic architecture that is typically regulated by the dynamic assembly of two hydrophobic spines that is usually mediated by the phosphorylation of an activation loop phosphate. Cyclic AMP-dependent protein kinase (protein kinase A (PKA)) is used as a prototype to exemplify these features of the PKA superfamily. Specificity in PKA signalling is achieved in large part by packaging the enzyme as inactive tetrameric holoenzymes with regulatory subunits that then are localized to macromolecular complexes in close proximity to dedicated substrates by targeting scaffold proteins. In this way, the cell creates discrete foci that most likely represent the physiological environment for cyclic AMP-mediated signalling.
Figures




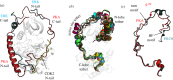

References
-
- Krebs E. G., Fischer E. H. 1956. The phosphorylase-B to phosphorylase-A converting enzyme of rabbit skeletal muscle. Biochim. Biophys. Acta 20, 150–157 10.1016/0006-3002(56)90273-6 (doi:10.1016/0006-3002(56)90273-6) - DOI - PubMed
-
- Krebs E. G., Graves D. J., Fischer E. H. 1959. Factors affecting the activity of muscle phosphorylase B kinase. J. Biol. Chem. 234, 2867–2873 - PubMed
-
- Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. 2002. The protein kinase complement of the human genome. Science 298, 1912–1934 10.1126/science.1075762 (doi:10.1126/science.1075762) - DOI - PubMed
-
- Taylor S. S., Kornev A. P. 2011. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem. Sci. 36, 65–77 10.1016/j.tibs.2010.09.006 (doi:10.1016/j.tibs.2010.09.006) - DOI - PMC - PubMed
-
- Davoren P. R., Sutherland E. W. 1963. The cellular location of adenyl cyclase in the pigeon erythrocyte. J. Biol. Chem. 238, 3016–3023 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
