Modular evolution of phosphorylation-based signalling systems
- PMID: 22889906
- PMCID: PMC3415845
- DOI: 10.1098/rstb.2012.0106
Modular evolution of phosphorylation-based signalling systems
Abstract
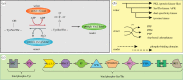
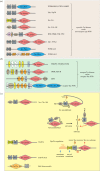
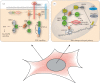
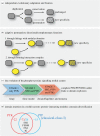
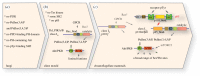
Phosphorylation sites are formed by protein kinases ('writers'), frequently exert their effects following recognition by phospho-binding proteins ('readers') and are removed by protein phosphatases ('erasers'). This writer-reader-eraser toolkit allows phosphorylation events to control a broad range of regulatory processes, and has been pivotal in the evolution of new functions required for the development of multi-cellular animals. The proteins that comprise this system of protein kinases, phospho-binding targets and phosphatases are typically modular in organization, in the sense that they are composed of multiple globular domains and smaller peptide motifs with binding or catalytic properties. The linkage of these binding and catalytic modules in new ways through genetic recombination, and the selection of particular domain combinations, has promoted the evolution of novel, biologically useful processes. Conversely, the joining of domains in aberrant combinations can subvert cell signalling and be causative in diseases such as cancer. Major inventions such as phosphotyrosine (pTyr)-mediated signalling that flourished in the first multi-cellular animals and their immediate predecessors resulted from stepwise evolutionary progression. This involved changes in the binding properties of interaction domains such as SH2 and their linkage to new domain types, and alterations in the catalytic specificities of kinases and phosphatases. This review will focus on the modular aspects of signalling networks and the mechanism by which they may have evolved.
Figures

References
-
- Cohen P. 2002. The origins of protein phosphorylation. Nat. Cell Biol. 4, E127–E130 10.1038/ncb0502-e127 (doi:10.1038/ncb0502-e127) - DOI - PubMed
-
- Hunter T. 2000. Signaling: 2000 and beyond. Cell 100, 113–127 10.1016/S0092-8674(00)81688-8 (doi:10.1016/S0092-8674(00)81688-8) - DOI - PubMed
-
- Cozzone A. J. 1988. Protein phosphorylation in prokaryotes. Annu. Rev. Microbiol. 42, 97–125 10.1146/annurev.mi.42.100188.000525 (doi:10.1146/annurev.mi.42.100188.000525) - DOI - PubMed
-
- Ciesla J., Fraczyk T., Rode W. 2011. Phosphorylation of basic amino acid residues in proteins: important but easily missed. Acta Biochim. Polonica 58, 137–148 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
