Mutational properties of amino acid residues: implications for evolvability of phosphorylatable residues
- PMID: 22889909
- PMCID: PMC3415843
- DOI: 10.1098/rstb.2012.0076
Mutational properties of amino acid residues: implications for evolvability of phosphorylatable residues
Erratum in
- Philos Trans R Soc Lond B Biol Sci. 2012 Nov 5;367(1602):3058
Abstract
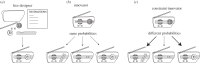
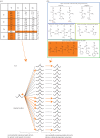
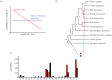
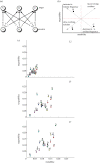
As François Jacob pointed out over 30 years ago, evolution is a tinkering process, and, as such, relies on the genetic diversity produced by mutation subsequently shaped by Darwinian selection. However, there is one implicit assumption that is made when studying this tinkering process; it is typically assumed that all amino acid residues are equally likely to mutate or to result from a mutation. Here, by reconstructing ancestral sequences and computing mutational probabilities for all the amino acid residues, we refute this assumption and show extensive inequalities between different residues in terms of their mutational activity. Moreover, we highlight the importance of the genetic code and physico-chemical properties of the amino acid residues as likely causes of these inequalities and uncover serine as a mutational hot spot. Finally, we explore the consequences that these different mutational properties have on phosphorylation site evolution, showing that a higher degree of evolvability exists for phosphorylated threonine and, to a lesser extent, serine in comparison with tyrosine residues. As exemplified by the suppression of serine's mutational activity in phosphorylation sites, our results suggest that the cell can fine-tune the mutational activities of amino acid residues when they reside in functional protein regions.
Figures

References
-
- Jacob F. 1977. Evolution and tinkering. Science 196, 1161–1166 10.1126/science.860134 (doi:10.1126/science.860134) - DOI - PubMed
-
- Pastor-Satorras R., Smith E., Solé R. V. 2003. Evolving protein interaction networks through gene duplication. J. Theor. Biol. 222, 199–210 10.1016/S0022-5193(03)00028-6 (doi:10.1016/S0022-5193(03)00028-6) - DOI - PubMed
-
- Dayhoff M. O., Schwartz R., Orcutt B. C. 1978. A model of evolutionary change in proteins. In Atlas of protein sequence and structure, vol. 5, suppl. 3 (ed. Dayhoff M. O.), pp. 345–352 Washington, DC: National Biomedical Research Foundation
-
- Henikoff S., Henikoff J. G. 1992. Amino acid substitution matrices from protein blocks. Proc. Natl Acad. Sci. USA 89, 109 15–109 19. 10.1073/pnas.89.22.10915 (doi:10.1073/pnas.89.22.10915) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
