The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review
- PMID: 22890029
- PMCID: PMC3418219
- DOI: 10.1136/bmj.e5182
The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review
Abstract
Objective: To indirectly compare the effectiveness of ranibizumab and bevacizumab in the treatment of diabetic macular oedema.
Design: Systematic review and indirect comparison.
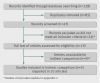
Data sources: Medline (1996-September 2011), Embase (1996-September 2011), and the Cochrane Central Register of Controlled Trials (Issue 4, 2011).
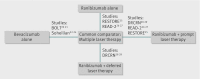
Selection criteria for studies: Randomised trials evaluating ranibizumab or bevacizumab in diabetic macular oedema with a common comparator and sufficient methodological similarity to be included within an indirect comparison were eligible for inclusion.
Main outcome measures: The primary outcome was the proportion of patients with an improvement in best corrected visual acuity of more than two lines on the Early Treatment Diabetic Retinopathy Study (ETDRS) scale. Secondary outcomes included mean changes in best corrected visual acuity and in central macular thickness, and adverse events. Best corrected visual acuity was converted to logMAR units, a linear scale of visual acuity with positive values representing increasing visual loss. Indirect comparisons were done using Bayesian methods to estimate relative treatment effects of bevacizumab and ranibizumab.
Results: Five randomised controlled trials with follow-up of 6-12 months and a common comparator (multiple laser treatment) were sufficiently similar to be included in the indirect comparison. Generally studies were small, resulting in wide credible intervals. The proportions of patients with an improvement in best corrected visual acuity of >2 lines were 21/77 participants (27%) for bevacizumab and 60/152 participants (39%) for ranibizumab (odds ratio 0.95 (95% credible interval 0.23 to 4.32)). The wide credible intervals cannot exclude a greater improvement, or worse outcome, for either drug. The mean change in best corrected visual acuity non-significantly favoured bevacizumab (treatment effect -0.08 logMAR units (-0.19 to 0.04)). The difference in mean change in central macular thickness was not statistically significant between ranibizumab and bevacizumab (treatment effect -6.9 μm (-88.5 to 65.4)).
Conclusions: Results suggest no difference in effectiveness between bevacizumab and ranibizumab, but the wide credible intervals cannot exclude the possibility that either drug might be superior. Sufficiently powered, direct head to head trials are needed.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond) 2004;18:963-83. - PubMed
-
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 2003;19:442-55. - PubMed
-
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985;103:1796-806. - PubMed
-
- Lee CM, Olk RJ. Modified grid laser photocoagulation for diffuse diabetic macular edema. Long term visual results. Ophthalmology 1991;98:1594-604 - PubMed
-
- Simo R, Hernandez C. Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia 2008;51:1574-80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical