Oxidative stress triggers Ca-dependent lysosome trafficking and activation of acid sphingomyelinase
- PMID: 22890197
- PMCID: PMC3777434
- DOI: 10.1159/000341460
Oxidative stress triggers Ca-dependent lysosome trafficking and activation of acid sphingomyelinase
Abstract
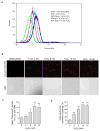
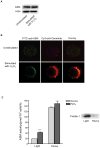
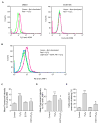
Recent studies demonstrate that rapid translocation of the acid sphingomyelinase (ASM), a lysosomal hydrolase, to the outer leaflet of the cell membrane and concomitant release of ceramide constitute a common cellular signaling cascade to various stimuli including CD95 ligation, UV-irradiation, bacterial and viral infections. Reactive oxygen species (ROS) were shown to play a crucial role in regulating this signaling cascade at least for some bacterial infections and UV-irradiation. However, the precise role of ROS for regulation of ASM is unknown. Here, by confocal microscopy and flow cytometry analysis, we demonstrate that hydrogen peroxide (H(2)O(2)), a primary form of ROS in mammalian cells, induces very rapid translocation of ASM and formation of ceramide-enriched membrane platforms in the plasma membrane of Jurkat T cells. In parallel, H(2)O(2) triggers lysosome trafficking and fusion with the plasma membrane, i.e. lysosome exocytosis, as detected by exposure of a lysosome-associated protein, LAMP1. Depletion of intracellular Ca(2+) by cell permeable EGTA-AM inhibits H(2)O(2)-induced lysosome exocytosis, ASM translocation and formation of ceramide-enriched platforms. Pharmacological inhibition or genetic deficiency of ASM did not affect H(2)O(2)-induced lysosome exocytosis. These results indicate that ROS-induced membrane translocation of ASM is mediated by exocytosis of lysosomes, which is dependent on intracellular Ca(2+) release.
Copyright © 2012 S. Karger AG, Basel.
Figures


References
-
- Levade T, Jaffrezou JP. Signalling sphingomyelinases: Which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. - PubMed
-
- McGovern MM, Pohl-Worgall T, Deckelbaum RJ, Simpson W, Mendelson D, Desnick RJ, Schuchman EH, Wasserstein MP. Lipid abnormalities in children with types a and b niemann pick disease. J Pediatr. 2004;145:77–81. - PubMed
-
- McGovern MM, Aron A, Brodie SE, Desnick RJ, Wasserstein MP. Natural history of type a niemann-pick disease: Possible endpoints for therapeutic trials. Neurology. 2006;66:228–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
