Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry
- PMID: 22890223
- PMCID: PMC3438468
- DOI: 10.1038/ajg.2012.218
Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry
Abstract
Objectives: The objective of this study was to contribute long-term safety data for infliximab and other therapies in Crohn's disease (CD).
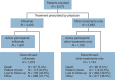
Methods: We prospectively evaluated CD patients enrolled in the large, observational Crohn's Therapy, Resource, Evaluation, and Assessment Tool registry, established to compare infliximab safety with conventional nonbiological medications in CD.
Results: A total of 6,273 patients were enrolled and evaluated on or before 23 February 2010; 3,420 received infliximab (17,712 patient-years; 89.9% received ≥ 2 infusions) and 2,853 received other-treatments-only (13,251 patient-years). Mean length of patient follow-up was 5.2 years. More infliximab- than other-treatments-only-treated patients had moderate-to-severe (30.6% vs. 10.7%) or severe-to-fulminant (2.5% vs. 0.6%) disease severity (P < 0.001). In the year before enrollment, more infliximab- than other-treatments-only-treated patients required surgical intervention (17.4% vs. 13.6%), medical hospitalization (14.2% vs. 8.8%), prednisone (47.8% vs. 31.4%), immunomodulators (52.0% vs. 32.1%), and narcotic analgesics (17.3% vs. 9.1%). Patient mortality was similar for infliximab- and other-treatments-only-treated patients (0.58 vs. 0.59/100 patient-years). In multivariate logistic regression analyses, treatment with prednisone (hazard ratio (HR) = 2.14, 95% confidence interval (CI) = 1.55, 2.95; P < 0.001) or narcotic analgesics (HR = 1.79, 95% CI = 1.29, 2.48; P < 0.001) and age (HR = 1.08, 95% CI = 1.07, 1.09; P < 0.001) were associated with increased mortality risk. Neither infliximab nor immunomodulator treatment was associated with increased mortality risk. Factors independently associated with serious infections included moderate-to-severe disease activity (HR = 2.24, 95% CI = 1.57, 3.19; P < 0.001), narcotic analgesic treatment (HR = 1.98, 95% CI = 1.44, 2.73; P < 0.001), prednisone therapy (HR = 1.57, 95% CI = 1.17, 2.10; P = 0.002), and infliximab treatment (HR = 1.43, 95% CI = 1.11, 1.84; P = 0.006).
Conclusions: Mortality was similar between infliximab- and other-treatments-only-treated CD patients. An increased risk of serious infection with infliximab was observed, although CD severity and use of prednisone or narcotic analgesics carried higher risks.
Figures
Comment in
-
What to take from TREAT?Am J Gastroenterol. 2012 Sep;107(9):1423-5. doi: 10.1038/ajg.2012.224. Am J Gastroenterol. 2012. PMID: 22951879
-
Cytomegalovirus pneumonia: a possible cause of death in patients with Crohn's disease.Am J Gastroenterol. 2013 Mar;108(3):454. doi: 10.1038/ajg.2012.431. Am J Gastroenterol. 2013. PMID: 23459056 No abstract available.
References
-
- Lee TW, Fedorak RN. Tumor necrosis factor-α monoclonal antibodies in the treatment of inflammatory bowel disease: clinical practice pharmacology. Gastroenterol Clin North Am. 2010;39:543–557. - PubMed
-
- Oussalah A, Danese S, Peyrin-Biroulet L. Efficacy of TNF antagonists beyond one year in adult and pediatric inflammatory bowel diseases: a systematic review. Curr Drug Targets. 2010;11:156–175. - PubMed
-
- Lin MV, Blonski W, Lichtenstein GR. What is the optimal therapy for Crohn's disease: step-up or top-down. Expert Rev Gastroenterol Hepatol. 2010;4:167–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

