The vesicular glutamate transporter VGLUT3 contributes to protection against neonatal hypoxic stress
- PMID: 22890712
- PMCID: PMC3497571
- DOI: 10.1113/jphysiol.2012.230722
The vesicular glutamate transporter VGLUT3 contributes to protection against neonatal hypoxic stress
Abstract
Neonates respond to hypoxia initially by increasing ventilation, and then by markedly decreasing both ventilation (hypoxic ventilatory decline) and oxygen consumption (hypoxic hypometabolism). This latter process, which vanishes with age, reflects a tight coupling between ventilatory and thermogenic responses to hypoxia. The neurological substrate of hypoxic hypometabolism is unclear, but it is known to be centrally mediated, with a strong involvement of the 5-hydroxytryptamine (5-HT, serotonin) system. To clarify this issue, we investigated the possible role of VGLUT3, the third subtype of vesicular glutamate transporter. VGLUT3 contributes to glutamate signalling by 5-HT neurons, facilitates 5-HT transmission and is expressed in strategic regions for respiratory and thermogenic control. We therefore assumed that VGLUT3 might significantly contribute to the response to hypoxia. To test this possibility, we analysed this response in newborn mice lacking VGLUT3 using anatomical, biochemical, electrophysiological and integrative physiology approaches. We found that the lack of VGLUT3 did not affect the histological organization of brainstem respiratory networks or respiratory activity under basal conditions. However, it impaired respiratory responses to 5-HT and anoxia, showing a marked alteration of central respiratory control. These impairments were associated with altered 5-HT turnover at the brainstem level. Furthermore, under cold conditions, the lack of VGLUT3 disrupted the metabolic rate, body temperature, baseline breathing and the ventilatory response to hypoxia. We conclude that VGLUT3 expression is dispensable under basal conditions but is required for optimal response to hypoxic stress in neonates.
Figures
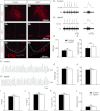
 ) (K), or number of apnoeas per 30 s period (L). ns: non significant (P > 0.05); Student's unpaired t test. Values shown are means ± SEM. See Tables 1 and 2 for full statistical analyses.
) (K), or number of apnoeas per 30 s period (L). ns: non significant (P > 0.05); Student's unpaired t test. Values shown are means ± SEM. See Tables 1 and 2 for full statistical analyses.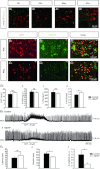

 ), tidal volume (VT) and breathing frequency (Rf). Vglut3−/−pups (n = 63) displayed decreased ventilation during both normoxia and hypoxia, compared to control pups (n = 200). Both groups displayed a biphasic response to hypoxia (shaded), with the initial increase in
), tidal volume (VT) and breathing frequency (Rf). Vglut3−/−pups (n = 63) displayed decreased ventilation during both normoxia and hypoxia, compared to control pups (n = 200). Both groups displayed a biphasic response to hypoxia (shaded), with the initial increase in  being followed by a marked decrease (under the control of VT and Rf). Ea–c, percentage change in breathing variables in response to hypoxia, relative to pre-hypoxic levels (hyperpnoeic response and hypoxic decline); HVD was significantly lower in Vglut3−/− pups. Dd, oxygen consumption (
being followed by a marked decrease (under the control of VT and Rf). Ea–c, percentage change in breathing variables in response to hypoxia, relative to pre-hypoxic levels (hyperpnoeic response and hypoxic decline); HVD was significantly lower in Vglut3−/− pups. Dd, oxygen consumption ( ) at 26°C under normoxia and in response to hypoxia (shaded) in 6-day-old Vglut3−/− (n = 7) and control pups (n = 29). The smaller
) at 26°C under normoxia and in response to hypoxia (shaded) in 6-day-old Vglut3−/− (n = 7) and control pups (n = 29). The smaller  in Vglut3−/− pups (P < 0.001) reflected their impaired thermogenesis. Ed, percentage change in
in Vglut3−/− pups (P < 0.001) reflected their impaired thermogenesis. Ed, percentage change in  relative to pre-hypoxic levels was smaller in Vglut3−/− pups. *P < 0.05; **P < 0.01; ***P < 0.001, Student's unpaired t test. Values shown are group means ± SEM. See Tables 1 and 2 for full statistical analyses.
relative to pre-hypoxic levels was smaller in Vglut3−/− pups. *P < 0.05; **P < 0.01; ***P < 0.001, Student's unpaired t test. Values shown are group means ± SEM. See Tables 1 and 2 for full statistical analyses.References
-
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. - PMC - PubMed
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. - PubMed
-
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–1400. - PubMed
-
- Bollen B, Bouslama M, Matrot B, Rotrou Y, Vardon G, Lofaso F, Van den Bergh O, D’Hooge R, Gallego J. Cold stimulates the behavioral response to hypoxia in newborn mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1503–1511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

