Neurocardiac dysregulation and neurogenic arrhythmias in a transgenic mouse model of Huntington's disease
- PMID: 22890713
- PMCID: PMC3528995
- DOI: 10.1113/jphysiol.2012.238113
Neurocardiac dysregulation and neurogenic arrhythmias in a transgenic mouse model of Huntington's disease
Abstract
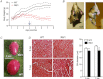
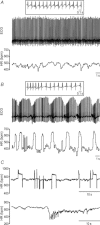
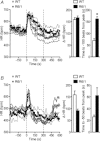
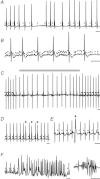
Huntington's disease (HD) is a heritable neurodegenerative disorder, with heart disease implicated as one major cause of death. While the responsible mechanism remains unknown, autonomic nervous system (ANS) dysfunction may play a role. We studied the cardiac phenotype in R6/1 transgenic mice at early (3 months old) and advanced (7 months old) stages of HD. While exhibiting a modest reduction in cardiomyocyte diameter, R6/1 mice had preserved baseline cardiac function. Conscious ECG telemetry revealed the absence of 24-h variation of heart rate (HR), and higher HR levels than wild-type littermates in young but not older R6/1 mice. Older R6/1 mice had increased plasma level of noradrenaline (NA), which was associated with reduced cardiac NA content. R6/1 mice also had unstable R-R intervals that were reversed following atropine treatment, suggesting parasympathetic nervous activation, and developed brady- and tachyarrhythmias, including paroxysmal atrial fibrillation and sudden death. c-Fos immunohistochemistry revealed greater numbers of active neurons in ANS-regulatory regions of R6/1 brains. Collectively, R6/1 mice exhibit profound ANS-cardiac dysfunction involving both sympathetic and parasympathetic limbs, that may be related to altered central autonomic pathways and lead to cardiac arrhythmias and sudden death.
Figures





Similar articles
-
Longitudinal analysis of the electroencephalogram and sleep phenotype in the R6/2 mouse model of Huntington's disease.Brain. 2013 Jul;136(Pt 7):2159-72. doi: 10.1093/brain/awt132. Brain. 2013. PMID: 23801738
-
Cardiac Dysautonomia in Huntington's Disease.J Huntingtons Dis. 2013;2(3):251-61. doi: 10.3233/JHD-130054. J Huntingtons Dis. 2013. PMID: 25062674 Review.
-
Oral treatment with herbal formula B307 alleviates cardiac failure in aging R6/2 mice with Huntington's disease via suppressing oxidative stress, inflammation, and apoptosis.Clin Interv Aging. 2015 Jul 20;10:1173-87. doi: 10.2147/CIA.S86493. eCollection 2015. Clin Interv Aging. 2015. PMID: 26229452 Free PMC article.
-
Corticosterone dysregulation exacerbates disease progression in the R6/2 transgenic mouse model of Huntington's disease.Exp Neurol. 2016 Sep;283(Pt A):308-17. doi: 10.1016/j.expneurol.2016.06.028. Epub 2016 Jul 2. Exp Neurol. 2016. PMID: 27381424 Free PMC article.
-
The role of the autonomic nervous system in arrhythmias and sudden cardiac death.Auton Neurosci. 2017 Jul;205:1-11. doi: 10.1016/j.autneu.2017.03.005. Epub 2017 Mar 31. Auton Neurosci. 2017. PMID: 28392310 Review.
Cited by
-
Systemic manifestation and contribution of peripheral tissues to Huntington's disease pathogenesis.Ageing Res Rev. 2021 Aug;69:101358. doi: 10.1016/j.arr.2021.101358. Epub 2021 May 9. Ageing Res Rev. 2021. PMID: 33979693 Free PMC article. Review.
-
Nitric oxide dysregulation in platelets from patients with advanced Huntington disease.PLoS One. 2014 Feb 25;9(2):e89745. doi: 10.1371/journal.pone.0089745. eCollection 2014. PLoS One. 2014. PMID: 24587005 Free PMC article.
-
Rosiglitazone Ameliorates Cardiac and Skeletal Muscle Dysfunction by Correction of Energetics in Huntington's Disease.Cells. 2022 Aug 27;11(17):2662. doi: 10.3390/cells11172662. Cells. 2022. PMID: 36078070 Free PMC article.
-
Circadian dysfunction in the Q175 model of Huntington's disease: Network analysis.J Neurosci Res. 2019 Dec;97(12):1606-1623. doi: 10.1002/jnr.24505. Epub 2019 Jul 29. J Neurosci Res. 2019. PMID: 31359503 Free PMC article.
-
Sleep and Circadian Rhythm Dysfunction in Animal Models of Huntington's Disease.J Huntingtons Dis. 2023;12(2):133-148. doi: 10.3233/JHD-230574. J Huntingtons Dis. 2023. PMID: 37334613 Free PMC article. Review.
References
-
- Arora R, Ulphani JS, Villuendas R, Ng J, Harvey L, Thordson S, Inderyas F, Lu Y, Gordon D, Denes P, Greene R, Crawford S, Decker R, Morris A, Goldberger J, Kadish AH. Neural substrate for atrial fibrillation: implications for targeted parasympathetic blockade in the posterior left atrium. Am J Physiol Heart Circ Physiol. 2008;294:H134–H144. - PubMed
-
- Aziz NA, Anguelova GV, Marinus J, van Dijk JG, Roos RA. Autonomic symptoms in patients and pre-manifest mutation carriers of Huntington's disease. Eur J Neurol. 2010;17:1068–1074. - PubMed
-
- Bar KJ, Boettger MK, Andrich J, Epplen JT, Fischer F, Cordes J, Koschke M, Agelink MW. Cardiovagal modulation upon postural change is altered in Huntington's disease. Eur J Neurol. 2008;15:869–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

