Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration
- PMID: 22890761
- PMCID: PMC3486529
- DOI: 10.1128/AAC.00802-12
Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration
Abstract
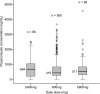
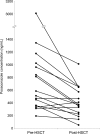
Posaconazole has an important role in the prophylaxis and salvage treatment of invasive fungal infections (IFIs), although poor and variable bioavailability remains an important clinical concern. Therapeutic drug monitoring of posaconazole concentrations has remained contentious, with the use of relatively small patient cohorts in previous studies hindering the assessment of exposure-response relationships. This multicenter retrospective study aimed to investigate relationships between posaconazole concentration and clinical outcomes and adverse events and to assess clinical factors and drug interactions that may affect posaconazole concentrations. Medical records were reviewed for patients who received posaconazole and had ≥1 concentration measured at six hospitals in Australia. Data from 86 patients with 541 posaconazole concentrations were included in the study. Among 72 patients taking posaconazole for prophylaxis against IFIs, 12 patients (17%) developed a breakthrough fungal infection; median posaconazole concentrations were significantly lower than in those who did not develop fungal infection (median [range], 289 [50 to 471] ng/ml versus 485 [0 to 2,035] ng/ml; P < 0.01). The median posaconazole concentration was a significant predictor of breakthrough fungal infection via binary logistic regression (P < 0.05). A multiple linear regression analysis identified a number of significant drug interactions associated with reduced posaconazole exposure, including coadministration with proton pump inhibitors, metoclopramide, phenytoin or rifampin, and the H(2) antagonist ranitidine (P < 0.01). Clinical factors such as mucositis, diarrhea, and the early posttransplant period in hematopoietic stem cell transplant recipients were also associated with reduced posaconazole exposure (P < 0.01). Low posaconazole concentrations are common and are associated with breakthrough fungal infection, supporting the utility of monitoring posaconazole concentrations to ensure optimal systemic exposure.
Figures


Comment in
-
What types of Candida infections should be included when evaluating breakthrough infections during posaconazole prophylaxis?Antimicrob Agents Chemother. 2013 Jun;57(6):2905. doi: 10.1128/AAC.00189-13. Antimicrob Agents Chemother. 2013. PMID: 23674753 Free PMC article. No abstract available.
-
Reply to "What types of Candida infections should be included when evaluating breakthrough infections during posaconazole prophylaxis?".Antimicrob Agents Chemother. 2013 Jun;57(6):2906. doi: 10.1128/AAC.00592-13. Antimicrob Agents Chemother. 2013. PMID: 23674754 Free PMC article. No abstract available.
References
-
- Anderson GD. 2004. Pharmacogenetics and enzyme induction/inhibition properties of antiepileptic drugs. Neurology 63:S3–S8 - PubMed
-
- Bryant AM, Slain D, Cumpston A, Craig M. 2011. A post-marketing evaluation of posaconazole plasma concentrations in neutropenic patients with haematological malignancy receiving posaconazole prophylaxis. Int. J. Antimicrob. Agents 37:266–269 - PubMed
-
- Centers for Disease Control and Prevention, Infectious Disease Society of America, and American Society of Blood and Marrow Transplantation 2000. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm. Rep. 49(RR-10):1–128 - PubMed
-
- Chhun S, et al. 2007. Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 852:223–228 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

