Uptake and persistence of Mycobacterium avium subsp. paratuberculosis in human monocytes
- PMID: 22890992
- PMCID: PMC3486062
- DOI: 10.1128/IAI.00534-12
Uptake and persistence of Mycobacterium avium subsp. paratuberculosis in human monocytes
Abstract
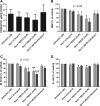
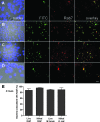
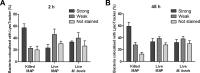
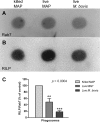
Mycobacterium avium subsp. paratuberculosis is a bacterium sometimes found in human blood and tissue samples that may have a role in the etiology of Crohn's disease in humans. To date, however, there have been few studies examining the interactions of these bacteria with human cells. Using the THP-1 human monocytic cell line, this study shows that the uptake and trafficking of M. avium subsp. paratuberculosis in human cells are cholesterol dependent and that these bacteria localize to cholesterol-rich compartments that are slow to acidify. M. avium subsp. paratuberculosis bacteria containing phagosomes stain for the late endosomal marker Rab7, but recruitment of the Rab7-interacting lysosomal protein that regulates the fusion of bacterium-containing phagosomes with lysosomal compartments and facilitates subsequent bacterial clearance is significantly reduced. Disruption of phagosome acidification via this mechanism may contribute to M. avium subsp. paratuberculosis persistence in human cells, but there was no evidence that internalized M. avium subsp. paratuberculosis also affects the survival of bacteria taken up during a secondary phagocytic event.
Figures


Similar articles
-
Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria.Cell Microbiol. 2001 Aug;3(8):551-66. doi: 10.1046/j.1462-5822.2001.00139.x. Cell Microbiol. 2001. PMID: 11488816
-
Altered Toll-like receptor 9 signaling in Mycobacterium avium subsp. paratuberculosis-infected bovine monocytes reveals potential therapeutic targets.Infect Immun. 2013 Jan;81(1):226-37. doi: 10.1128/IAI.00785-12. Epub 2012 Oct 31. Infect Immun. 2013. PMID: 23115040 Free PMC article.
-
Bovine monocyte TLR2 receptors differentially regulate the intracellular fate of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium.J Leukoc Biol. 2008 Jan;83(1):48-55. doi: 10.1189/jlb.0707490. Epub 2007 Oct 3. J Leukoc Biol. 2008. PMID: 17913973
-
Role of the mitogen-activated protein kinase pathway in the differential response of bovine monocytes to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium.Microbes Infect. 2007 Nov-Dec;9(14-15):1545-52. doi: 10.1016/j.micinf.2007.08.008. Epub 2007 Sep 2. Microbes Infect. 2007. PMID: 18035573
-
[Mycobacterium avium subsp. paratuberculosis in food and its relationship with Crohn's disease].Rev Argent Microbiol. 2007 Jan-Mar;39(1):57-68. Rev Argent Microbiol. 2007. PMID: 17585661 Review. Spanish.
Cited by
-
Randomized, Double-Blind, Placebo-Controlled Study of Anti-Mycobacterial Therapy (RHB-104) in Active Crohn's Disease.Antibiotics (Basel). 2024 Jul 25;13(8):694. doi: 10.3390/antibiotics13080694. Antibiotics (Basel). 2024. PMID: 39199994 Free PMC article.
-
Mammalian cell entry operons; novel and major subset candidates for diagnostics with special reference to Mycobacterium avium subspecies paratuberculosis infection.Vet Q. 2019 Dec;39(1):65-75. doi: 10.1080/01652176.2019.1641764. Vet Q. 2019. PMID: 31282842 Free PMC article. Review.
-
Mapping Crohn's Disease Pathogenesis with Mycobacterium paratuberculosis: A Hijacking by a Stealth Pathogen.Dig Dis Sci. 2024 Jul;69(7):2289-2303. doi: 10.1007/s10620-024-08508-4. Epub 2024 Jun 19. Dig Dis Sci. 2024. PMID: 38896362 Review.
-
Mycobacterium paratuberculosis zoonosis is a One Health emergency.Ecohealth. 2022 Jun;19(2):164-174. doi: 10.1007/s10393-022-01602-x. Epub 2022 Jun 2. Ecohealth. 2022. PMID: 35655048 Free PMC article. Review.
-
Vitamin D3 alters macrophage phenotype and endosomal trafficking markers in dairy cattle naturally infected with Mycobacterium avium subsp. paratuberculosis.Front Cell Infect Microbiol. 2022 Oct 5;12:1021657. doi: 10.3389/fcimb.2022.1021657. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36275033 Free PMC article.
References
-
- Bentley RW, et al. 2008. Incidence of Mycobacterium avium subspecies paratuberculosis in a population-based cohort of patients with Crohn's disease and control subjects. Am. J. Gastroenterol. 103:1168–1172 - PubMed
-
- Cheville NF, et al. 2001. Intracellular trafficking of Mycobacterium avium ss. paratuberculosis in macrophages. Dtsch. Tierarztl. Wochenschr. 108:236–243 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

