The nature and influence of pharmaceutical industry involvement in asthma trials
- PMID: 22891187
- PMCID: PMC3411392
- DOI: 10.1155/2012/890457
The nature and influence of pharmaceutical industry involvement in asthma trials
Abstract
Background: Pharmaceutical industry-sponsored research has been shown to be biased toward reporting positive results. Frequent industry participation in trials assessing the efficacy of inhaled corticosteroid (ICS) and long-acting beta<span style="vertical-align: sub">2<⁄span>-agonist (LABA) combination treatment makes assessing industry influence difficult and warrants an assessment of specific potential publication bias in this area.
Objective: To describe the frequency of industry involvement in ICS⁄LABA trials and explore associations among significant outcomes, type of industry involvement and type of primary outcome.
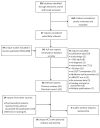
Methods: A systematic review of trials comparing ICS⁄LABA combination therapy with ICS monotherapy for asthma was conducted. Data concerning the type of industry sponsorship, primary outcome and statistical results were collected. Comparisons between type of sponsorship and significant results were analyzed using Pearson's chi squared test and relative risk.
Results: Of 91 included studies (median year of publication 2005 [interquartile range 1994 to 2008]), 86 (95%) reported pharmaceutical involvement. Author affiliation was reported in 49 of 86 (57%), and 19 of 86 (22%) were industry-reported trials without full publications. The remainder were published journal articles. Studies with a first or senior author affiliated with industry were 1.5 times more likely to report statistically significant results for the primary outcome compared with studies with other types of industry involvement. Pulmonary measures were 1.5 times more likely to be statistically significant than were measures of asthma control.
Conclusions: The potential biases identified were consistent with other research focused on author role and industry involvement, and suggest that degree of bias may vary with type of affiliation.
HISTORIQUE :: Il a été démontré que la recherche commanditée par l’industrie pharmaceutique a un parti pris pour des résultats positifs. En raison de la participation fréquente de l’industrie à des essais évaluant l’efficacité de la bithérapie de corticoïdes en aérosol (CEA) et de bêta2-agonistes de longue durée (BALD), il est difficile d’évaluer l’influence de l’industrie, ce qui justifie une évaluation des partis pris potentiels précis à ce sujet dans les publications.
OBJECTIF :: Décrire la fréquence de la participation de l’industrie aux essais des CEA-BALD et explorer les associations entre les issues significatives, le type de participation de l’industrie et le type d’issues primaires.
MÉTHODOLOGIE :: Les chercheurs ont effectué une analyse systématique des essais comparant la bithérapie de CEA-BALD à une monothérapie aux CEA contre l’asthme. Ils ont colligé les données portant sur le type de commandite de l’industrie, les issues cliniques et les résultats statistiques. Ils ont analysé les comparaisons entre le type de commandite et les résultats significatifs au moyen de la méthode du khi-carré de Pearson et du risque relatif.
RÉSULTATS :: Sur les 91 études incluses (année médiane de publication : 2005 [plage interquartile de 1994 à 2008]), 86 (95 %) indiquaient une participation pharmaceutique. L’affiliation des auteurs était indiquée dans 49 de ces 86 études (57 %), et 19 des 86 études (22 %) étaient des essais déclarés par l’industrie sans publications complètes. Les autres étaient des articles publiés dans des revues. Les études dont l’auteur principal ou un auteur chevronné était associé à l’industrie étaient 1,5 fois plus susceptibles de faire état de résultats statistiquement significatifs à l’égard de l’issue primaire que les études profitant d’autres types de participation de l’industrie. Les mesures pulmonaires étaient également 1,5 fois plus susceptibles d’être statistiquement significatives que les mesures de contrôle de l’asthme.
CONCLUSIONS :: Les partis pris potentiels confirmaient d’autres études portant sur le rôle de l’auteur et la participation de l’industrie et laissent croire que le degré de parti pris peut varier selon le type d’affiliation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical