Efficacy of fat-soluble vitamin supplementation in infants with biliary atresia
- PMID: 22891232
- PMCID: PMC3428752
- DOI: 10.1542/peds.2011-1423
Efficacy of fat-soluble vitamin supplementation in infants with biliary atresia
Abstract
Objective: Cholestasis predisposes to fat-soluble vitamin (FSV) deficiencies. A liquid multiple FSV preparation made with tocopheryl polyethylene glycol-1000 succinate (TPGS) is frequently used in infants with biliary atresia (BA) because of ease of administration and presumed efficacy. In this prospective multicenter study, we assessed the prevalence of FSV deficiency in infants with BA who received this FSV/TPGS preparation.
Methods: Infants received FSV/TPGS coadministered with additional vitamin K as routine clinical care in a randomized double-blinded, placebo-controlled trial of corticosteroid therapy after hepatoportoenterostomy (HPE) for BA (identifier NCT 00294684). Levels of FSV, retinol binding protein, total serum lipids, and total bilirubin (TB) were measured 1, 3, and 6 months after HPE.
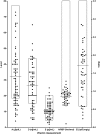
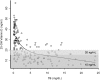
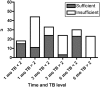
Results: Ninety-two infants with BA were enrolled in this study. Biochemical evidence of FSV insufficiency was common at all time points for vitamin A (29%-36% of patients), vitamin D (21%-37%), vitamin K (10%-22%), and vitamin E (16%-18%). Vitamin levels were inversely correlated with serum TB levels. Biochemical FSV insufficiency was much more common (15%-100% for the different vitamins) in infants whose TB was ≥2 mg/dL. At 3 and 6 months post HPE, only 3 of 24 and 0 of 23 infants, respectively, with TB >2 mg/dL were sufficient in all FSV.
Conclusions: Biochemical FSV insufficiency is commonly observed in infants with BA and persistent cholestasis despite administration of a TPGS containing liquid multiple FSV preparation. Individual vitamin supplementation and careful monitoring are warranted in infants with BA, especially those with TB >2 mg/dL.
Trial registration: ClinicalTrials.gov NCT00294684.
Figures
References
-
- Superina R, Magee JC, Brandt ML, et al. Childhood Liver Disease Research and Education Network . The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011;254(4):577–585 - PMC - PubMed
-
- Shneider BL, Brown MB, Haber B, et al. Biliary Atresia Research Consortium . A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148(4):467–474 - PubMed
-
- Sokol RJ, Heubi JE, Iannaccone S, Bove KE, Balistreri WF. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology. 1983;85(5):1172–1182 - PubMed
-
- Feranchak AP, Sokol RJ. Medical and nutritional management of cholestasis in infants and children. In: Suchy F, Balistreri W, Sokol R, eds. Liver Disease in Children. New York, NY: Cambridge University Press; 2007:190–231
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- DK 62470/DK/NIDDK NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- U01 DK062452/DK/NIDDK NIH HHS/United States
- U01 DK062466/DK/NIDDK NIH HHS/United States
- UL1RR024131/RR/NCRR NIH HHS/United States
- 5M01 RR00069/RR/NCRR NIH HHS/United States
- U01 DK062445/DK/NIDDK NIH HHS/United States
- DK 62481/DK/NIDDK NIH HHS/United States
- U24 DK062456/DK/NIDDK NIH HHS/United States
- UL1RR025005/RR/NCRR NIH HHS/United States
- U01 DK062481/DK/NIDDK NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- DK 62445/DK/NIDDK NIH HHS/United States
- DK 62452/DK/NIDDK NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- UL1RR025741/RR/NCRR NIH HHS/United States
- DK 62530/DK/NIDDK NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- DK 62497/DK/NIDDK NIH HHS/United States
- DK 62456/DK/NIDDK NIH HHS/United States
- U01 DK062453/DK/NIDDK NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- DK 62436/DK/NIDDK NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- UL1RR024992/RR/NCRR NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- DK 62453/DK/NIDDK NIH HHS/United States
- U01 DK062436/DK/NIDDK NIH HHS/United States
- UL1RR024153/RR/NCRR NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UL1RR025780/RR/NCRR NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- DK 62500/DK/NIDDK NIH HHS/United States
- U01 DK062456/DK/NIDDK NIH HHS/United States
- TL1 RR029877/RR/NCRR NIH HHS/United States
- UL1RR024134/RR/NCRR NIH HHS/United States
- U01 DK062500/DK/NIDDK NIH HHS/United States
- U01 DK062497/DK/NIDDK NIH HHS/United States
- UL1 RR026314/RR/NCRR NIH HHS/United States
- UL1RR026314/RR/NCRR NIH HHS/United States
- UL1RR029877/RR/NCRR NIH HHS/United States
- DK 62466/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

