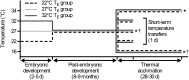
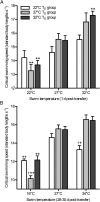
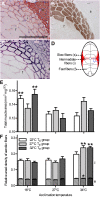
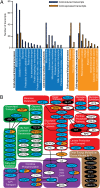
Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish
- PMID: 22891320
- PMCID: PMC3435178
- DOI: 10.1073/pnas.1205012109
Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish
Abstract
Global warming is intensifying interest in the mechanisms enabling ectothermic animals to adjust physiological performance and cope with temperature change. Here we show that embryonic temperature can have dramatic and persistent effects on thermal acclimation capacity at multiple levels of biological organization. Zebrafish embryos were incubated until hatching at control temperature (T(E) = 27 °C) or near the extremes for normal development (T(E) = 22 °C or 32 °C) and were then raised to adulthood under common conditions at 27 °C. Short-term temperature challenge affected aerobic exercise performance (U(crit)), but each T(E) group had reduced thermal sensitivity at its respective T(E). In contrast, unexpected differences arose after long-term acclimation to 16 °C, when performance in the cold was ∼20% higher in both 32 °C and 22 °C T(E) groups compared with 27 °C T(E) controls. Differences in performance after acclimation to cold or warm (34 °C) temperatures were partially explained by variation in fiber type composition in the swimming muscle. Cold acclimation changed the abundance of 3,452 of 19,712 unique and unambiguously identified transcripts detected in the fast muscle using RNA-Seq. Principal components analysis differentiated the general transcriptional responses to cold of the 27 °C and 32 °C T(E) groups. Differences in expression were observed for individual genes involved in energy metabolism, angiogenesis, cell stress, muscle contraction and remodeling, and apoptosis. Therefore, thermal acclimation capacity is not fixed and can be modified by temperature during early development. Developmental plasticity may thus help some ectothermic organisms cope with the more variable temperatures that are expected under future climate-change scenarios.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schulte PM, Healy TM, Fangue NA. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol. 2011;51:691–702. - PubMed
-
- Anguilleta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford, UK: Oxford Univ Press; 2009.
-
- Somero GN. Comparative physiology: A “crystal ball” for predicting consequences of global change. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1–R14. - PubMed
-
- Pörtner HO, Farrell AP. Ecology. Physiology and climate change. Science. 2008;322:690–692. - PubMed
-
- Fry FEJ, Hart JS. Cruising speed of goldfish in relation to water temperature. J Fish Res Board Can. 1948;7:169–175.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

