Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis
- PMID: 22891322
- PMCID: PMC3435183
- DOI: 10.1073/pnas.1203834109
Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis
Erratum in
- Proc Natl Acad Sci U S A. 2012 Sep 11;109(37):15072
Abstract
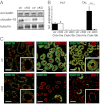
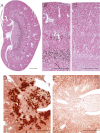
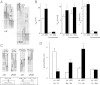
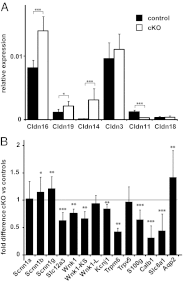
In the kidney, tight junction proteins contribute to segment specific selectivity and permeability of paracellular ion transport. In the thick ascending limb (TAL) of Henle's loop, chloride is reabsorbed transcellularly, whereas sodium reabsorption takes transcellular and paracellular routes. TAL salt transport maintains the concentrating ability of the kidney and generates a transepithelial voltage that drives the reabsorption of calcium and magnesium. Thus, functionality of TAL ion transport depends strongly on the properties of the paracellular pathway. To elucidate the role of the tight junction protein claudin-10 in TAL function, we generated mice with a deletion of Cldn10 in this segment. We show that claudin-10 determines paracellular sodium permeability, and that its loss leads to hypermagnesemia and nephrocalcinosis. In isolated perfused TAL tubules of claudin-10-deficient mice, paracellular permeability of sodium is decreased, and the relative permeability of calcium and magnesium is increased. Moreover, furosemide-inhibitable transepithelial voltage is increased, leading to a shift from paracellular sodium transport to paracellular hyperabsorption of calcium and magnesium. These data identify claudin-10 as a key factor in control of cation selectivity and transport in the TAL, and deficiency in this pathway as a cause of nephrocalcinosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Deletion of claudin-10 rescues claudin-16-deficient mice from hypomagnesemia and hypercalciuria.Kidney Int. 2018 Mar;93(3):580-588. doi: 10.1016/j.kint.2017.08.029. Epub 2017 Nov 10. Kidney Int. 2018. PMID: 29129401
-
Corticomedullary difference in the effects of dietary Ca²⁺ on tight junction properties in thick ascending limbs of Henle's loop.Pflugers Arch. 2016 Feb;468(2):293-303. doi: 10.1007/s00424-015-1748-7. Epub 2015 Oct 26. Pflugers Arch. 2016. PMID: 26497703
-
Claudin-16 and claudin-19 function in the thick ascending limb.Curr Opin Nephrol Hypertens. 2010 Sep;19(5):483-8. doi: 10.1097/MNH.0b013e32833b7125. Curr Opin Nephrol Hypertens. 2010. PMID: 20616717 Free PMC article. Review.
-
Function and regulation of claudins in the thick ascending limb of Henle.Pflugers Arch. 2009 May;458(1):77-88. doi: 10.1007/s00424-008-0589-z. Epub 2008 Sep 16. Pflugers Arch. 2009. PMID: 18795318 Free PMC article. Review.
-
Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport.Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):E219-E227. doi: 10.1073/pnas.1611684114. Epub 2016 Dec 27. Proc Natl Acad Sci U S A. 2017. PMID: 28028216 Free PMC article.
Cited by
-
Claudin-10 Expression Is Increased in Endometriosis and Adenomyosis and Mislocalized in Ectopic Endometriosis.Diagnostics (Basel). 2022 Nov 17;12(11):2848. doi: 10.3390/diagnostics12112848. Diagnostics (Basel). 2022. PMID: 36428908 Free PMC article.
-
HELIX Syndrome, a Claudinopathy with Relevant Dermatological Manifestations: Report of Two New Cases.Genes (Basel). 2024 May 26;15(6):687. doi: 10.3390/genes15060687. Genes (Basel). 2024. PMID: 38927623 Free PMC article.
-
One gene, two paracellular ion channels-claudin-10 in the kidney.Pflugers Arch. 2017 Jan;469(1):115-121. doi: 10.1007/s00424-016-1921-7. Epub 2016 Dec 10. Pflugers Arch. 2017. PMID: 27942952 Review.
-
A Novel Hypokalemic-Alkalotic Salt-Losing Tubulopathy in Patients with CLDN10 Mutations.J Am Soc Nephrol. 2017 Oct;28(10):3118-3128. doi: 10.1681/ASN.2016080881. Epub 2017 Jul 3. J Am Soc Nephrol. 2017. PMID: 28674042 Free PMC article.
-
Distinct cell types along thick ascending limb express pathways for monovalent and divalent cation transport.JCI Insight. 2025 Jun 5;10(13):e190992. doi: 10.1172/jci.insight.190992. eCollection 2025 Jul 8. JCI Insight. 2025. PMID: 40471686 Free PMC article.
References
-
- Greger R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflugers Arch. 1981;390:30–37. - PubMed
-
- Mineta K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–612. - PubMed
-
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

