Resistance to EGF receptor inhibitors in glioblastoma mediated by phosphorylation of the PTEN tumor suppressor at tyrosine 240
- PMID: 22891331
- PMCID: PMC3435194
- DOI: 10.1073/pnas.1211962109
Resistance to EGF receptor inhibitors in glioblastoma mediated by phosphorylation of the PTEN tumor suppressor at tyrosine 240
Abstract
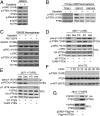
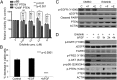
Glioblastoma multiforme (GBM) is the most aggressive of the astrocytic malignancies and the most common intracranial tumor in adults. Although the epidermal growth factor receptor (EGFR) is overexpressed and/or mutated in at least 50% of GBM cases and is required for tumor maintenance in animal models, EGFR inhibitors have thus far failed to deliver significant responses in GBM patients. One inherent resistance mechanism in GBM is the coactivation of multiple receptor tyrosine kinases, which generates redundancy in activation of phosphoinositide-3'-kinase (PI3K) signaling. Here we demonstrate that the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) tumor suppressor is frequently phosphorylated at a conserved tyrosine residue, Y240, in GBM clinical samples. Phosphorylation of Y240 is associated with shortened overall survival and resistance to EGFR inhibitor therapy in GBM patients and plays an active role in mediating resistance to EGFR inhibition in vitro. Y240 phosphorylation can be mediated by both fibroblast growth factor receptors and SRC family kinases (SFKs) but does not affect the ability of PTEN to antagonize PI3K signaling. These findings show that, in addition to genetic loss and mutation of PTEN, its modulation by tyrosine phosphorylation has important implications for the development and treatment of GBM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chronic activation of wild-type epidermal growth factor receptor and loss of Cdkn2a cause mouse glioblastoma formation.Cancer Res. 2011 Dec 1;71(23):7198-206. doi: 10.1158/0008-5472.CAN-11-1514. Epub 2011 Oct 10. Cancer Res. 2011. PMID: 21987724 Free PMC article.
-
Effects of epidermal growth factor receptor and phosphatase and tensin homologue gene expression on the inhibition of U87MG glioblastoma cell proliferation induced by protein kinase inhibitors.Clin Exp Pharmacol Physiol. 2013 Jan;40(1):13-21. doi: 10.1111/1440-1681.12026. Clin Exp Pharmacol Physiol. 2013. PMID: 23110505
-
Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells.Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15984-9. doi: 10.1073/pnas.1113416108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896743 Free PMC article.
-
Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance.Curr Cancer Drug Targets. 2012 Mar;12(3):197-209. doi: 10.2174/156800912799277557. Curr Cancer Drug Targets. 2012. PMID: 22268382 Free PMC article. Review.
-
Targeting EGFR and PI3K/mTOR pathways in glioblastoma: innovative therapeutic approaches.Med Oncol. 2025 Mar 10;42(4):97. doi: 10.1007/s12032-025-02652-1. Med Oncol. 2025. PMID: 40064710 Review.
Cited by
-
Fast metabolic response to drug intervention through analysis on a miniaturized, highly integrated molecular imaging system.J Nucl Med. 2013 Oct;54(10):1820-4. doi: 10.2967/jnumed.112.118497. Epub 2013 Aug 26. J Nucl Med. 2013. PMID: 23978446 Free PMC article.
-
Inhibition of Nuclear PTEN Tyrosine Phosphorylation Enhances Glioma Radiation Sensitivity through Attenuated DNA Repair.Cancer Cell. 2019 Mar 18;35(3):504-518.e7. doi: 10.1016/j.ccell.2019.01.020. Epub 2019 Feb 28. Cancer Cell. 2019. PMID: 30827889 Free PMC article.
-
Plasmatic membrane toll-like receptor expressions in human astrocytomas.PLoS One. 2018 Jun 18;13(6):e0199211. doi: 10.1371/journal.pone.0199211. eCollection 2018. PLoS One. 2018. PMID: 29912993 Free PMC article.
-
Inhibition of FoxO1 nuclear exclusion prevents metastasis of glioblastoma.Tumour Biol. 2014 Jul;35(7):7195-200. doi: 10.1007/s13277-014-1913-1. Epub 2014 Apr 27. Tumour Biol. 2014. Retraction in: Tumour Biol. 2017 Apr 20. doi: 10.1007/s13277-017-5487-6. PMID: 24771221 Retracted.
-
Paradoxes of the EphB1 receptor in malignant brain tumors.Cancer Cell Int. 2017 Feb 8;17:21. doi: 10.1186/s12935-017-0384-z. eCollection 2017. Cancer Cell Int. 2017. PMID: 28194092 Free PMC article. Review.
References
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Dey N, et al. The protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migration. Cancer Res. 2008;68:1862–1871. - PubMed
-
- Shen WH, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

