Endothelial Semaphorin 7A promotes neutrophil migration during hypoxia
- PMID: 22891341
- PMCID: PMC3435204
- DOI: 10.1073/pnas.1202165109
Endothelial Semaphorin 7A promotes neutrophil migration during hypoxia
Abstract
Recent studies identified basic biological principles that are shared by the immune and the nervous system. One of these analogies applies to the orchestration of cellular migration where guidance proteins that serve as a stop signal for axonal migration can also serve as a stop signal for the migration of immune-competent cells. The control of leukocyte migration is of key interest during conditions associated with inflammatory tissue changes such as tissue hypoxia or hypoxic inflammation. Semaphorins are members of these axon guidance molecules. Previously unknown, we report here the expression and induction of semaphorin 7A (SEMA7A) on endothelium through hypoxia-inducible factor 1α during hypoxia. This induction of SEMA7A translates into increased transmigration of polymorphonuclear neutrophil granulocytes across endothelial cells. Extension of these findings demonstrated an attenuated extravasation of polymorphonuclear neutrophil granulocytes in Sema7a-deficient mice from the vasculature during hypoxia. Studies using chimeric animals identified the expression of Sema7A on nonhematopoietic tissue to be the underlying cause of the observed results. Taken together, our findings demonstrate that neuronal guidance proteins do not only serve as a stop signal for leukocyte migration but also can propagate the extravasation of leukocytes from the vascular space. Future anti-inflammatory strategies might be based on this finding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
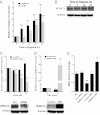
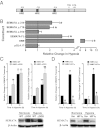
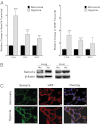
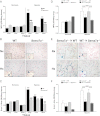
References
-
- Rosenberger P, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

