Diastereoselectivity in Lewis-acid-catalyzed Mukaiyama aldol reactions: a DFT study
- PMID: 22891640
- PMCID: PMC3465709
- DOI: 10.1021/ja3052975
Diastereoselectivity in Lewis-acid-catalyzed Mukaiyama aldol reactions: a DFT study
Abstract
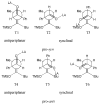
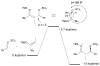
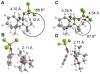
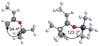
The basis for diastereoselectivity in Lewis-acid-catalyzed Mukaiyama aldol reactions was studied using density functional theory. By exploring the conformations of the transition structures for the diastereodifferentiating step of seven different reactions, simple models were generated. The effects of varying the substituents on the enol carbon and the α-carbon of the silyl enol ether from methyl to tert-butyl groups and the substituent on the aldehyde from methyl to phenyl groups were investigated by comparison of the transition structures for different reactions. Expanding on the previous qualitative models by Heathcock and Denmark, we found that while the pro-anti pathways take place via antiperiplanar transition structures, the pro-syn pathways prefer synclinal transition structures. The relative steric effects of the Lewis acid and trimethyl silyl groups and the influence of E/Z isomerism on the aldol transition state were investigated. By calculating 36 transition structures at the M06/6-311G*//B3LYP/6-31G* level of theory and employing the IEFPCM polarizable continuum model for solvation effects, this study expands the mechanistic knowledge and provides a model for understanding the diastereoselectivity in Lewis-acid-catalyzed Mukaiyama aldol reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

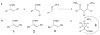


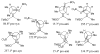
References
-
- Mukaiyama T, Narasaka K, Banno K. Chem Lett. 1973;2:1011–1014.
-
- Mukaiyama T, Banno K, Narasaka K. J Am Chem Soc. 1974;96:7503–7509.
-
- Saigo K, Osaki M, Mukaiyama T. Chem Lett. 1975;4:989–990.
-
- Gennari C, Bernardi A, Cardani S, Scolastico C. Tetrahedron Lett. 1985;26:797–800.
-
- Mahrwald R. Modern aldol reactions. Wiley-VCH; Weinheim: 2004. pp. 139–140.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

