Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4(+) /CD4(+) Foxp3(+) cells
- PMID: 22891805
- PMCID: PMC3482681
- DOI: 10.1111/imm.12006
Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4(+) /CD4(+) Foxp3(+) cells
Abstract
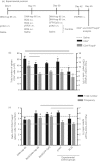
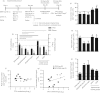
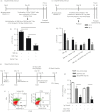
CD4(+) Foxp3(+) regulatory T cells inhibit the production of interferon-γ, which is the major mediator of protection against Mycobacterium tuberculosis infection. In this study, we evaluated whether the protection conferred by three different vaccines against tuberculosis was associated with the number of spleen and lung regulatory T cells. We observed that after homologous immunization with the 65 000 molecular weight heat-shock protein (hsp 65) DNA vaccine, there was a significantly higher number of spleen CD4(+) Foxp3(+) cells compared with non-immunized mice. Heterologous immunization using bacillus Calmette-Guérin (BCG) to prime and DNA-hsp 65 to boost (BCG/DNA-hsp 65) or BCG to prime and culture filtrate proteins (CFP)-CpG to boost (BCG/CFP-CpG) induced a significantly higher ratio of spleen CD4(+) /CD4(+) Foxp3(+) cells compared with non-immunized mice. In addition, the protection conferred by either the BCG/DNA-hsp 65 or the BCG/CFP-CpG vaccines was significant compared with the DNA-hsp 65 vaccine. Despite the higher ratio of spleen CD4(+) /CD4(+) Foxp3(+) cells found in BCG/DNA-hsp 65-immunized or BCG/CFP-CpG-immunized mice, the lungs of both groups of mice were better preserved than those of DNA-hsp 65-immunized mice. These results confirm the protective efficacy of BCG/DNA-hsp 65 and BCG/CFP-CpG heterologous prime-boost vaccines and the DNA-hsp 65 homologous vaccine. Additionally, the prime-boost regimens assayed here represent a promising strategy for the development of new vaccines to protect against tuberculosis because they probably induce a proper ratio of CD4(+) and regulatory (CD4(+) Foxp3(+) ) cells during the immunization regimen. In this study, this ratio was associated with a reduced number of regulatory cells and no injury to the lungs.
© 2012 The Authors. Immunology © 2012 Blackwell Publishing Ltd.
Figures

Similar articles
-
Mycobacterium tuberculosis culture filtrate proteins plus CpG Oligodeoxynucleotides confer protection to Mycobacterium bovis BCG-primed mice by inhibiting interleukin-4 secretion.Infect Immun. 2009 Dec;77(12):5311-21. doi: 10.1128/IAI.00580-09. Epub 2009 Sep 14. Infect Immun. 2009. PMID: 19752029 Free PMC article.
-
Vaccine-elicited 10-kilodalton culture filtrate protein-specific CD8+ T cells are sufficient to mediate protection against Mycobacterium tuberculosis infection.Infect Immun. 2008 May;76(5):2249-55. doi: 10.1128/IAI.00024-08. Epub 2008 Mar 10. Infect Immun. 2008. PMID: 18332205 Free PMC article.
-
Immunogenicity and protective efficacy of a DNA vaccine encoding the fusion protein of mycobacterium heat shock protein 65 (Hsp65) with human interleukin-2 against Mycobacterium tuberculosis in BALB/c mice.APMIS. 2008 Dec;116(12):1071-81. doi: 10.1111/j.1600-0463.2008.01095.x. APMIS. 2008. PMID: 19133010
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Next generation: tuberculosis vaccines that elicit protective CD8+ T cells.Expert Rev Vaccines. 2007 Jun;6(3):441-56. doi: 10.1586/14760584.6.3.441. Expert Rev Vaccines. 2007. PMID: 17542758 Free PMC article. Review.
Cited by
-
Evaluation of the overall IFN-γ and IL-17 pro-inflammatory responses after DNA therapy of tuberculosis.Hum Vaccin Immunother. 2013 May;9(5):1093-103. doi: 10.4161/hv.23417. Epub 2013 Jan 16. Hum Vaccin Immunother. 2013. PMID: 23324590 Free PMC article.
-
Bacillus Calmette-Guerin vaccine-mediated neuroprotection is associated with regulatory T-cell induction in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease.J Neurosci Res. 2013 Oct;91(10):1292-302. doi: 10.1002/jnr.23253. Epub 2013 Aug 1. J Neurosci Res. 2013. PMID: 23907992 Free PMC article.
-
Immunotherapeutic Activities of a DNA Plasmid Carrying the Mycobacterial hsp65 Gene (DNAhsp65).Front Med Technol. 2020 Dec 15;2:603690. doi: 10.3389/fmedt.2020.603690. eCollection 2020. Front Med Technol. 2020. PMID: 35047886 Free PMC article. Review.
-
Immunotherapeutic Effects of Different Doses of Mycobacterium tuberculosis ag85a/b DNA Vaccine Delivered by Electroporation.Front Immunol. 2022 May 4;13:876579. doi: 10.3389/fimmu.2022.876579. eCollection 2022. Front Immunol. 2022. PMID: 35603155 Free PMC article.
-
Regulatory T Cells in Mycobacterium tuberculosis Infection.Front Immunol. 2019 Sep 11;10:2139. doi: 10.3389/fimmu.2019.02139. eCollection 2019. Front Immunol. 2019. PMID: 31572365 Free PMC article. Review.
References
-
- Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004;4:408–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

