Rapid construction of the aza-propellane core of acutumine via a photochemical [2 + 2] cycloaddition reaction
- PMID: 22891873
- PMCID: PMC3445413
- DOI: 10.1021/ol3017963
Rapid construction of the aza-propellane core of acutumine via a photochemical [2 + 2] cycloaddition reaction
Abstract
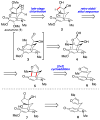
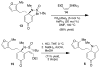
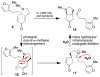
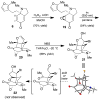
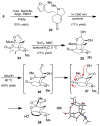
Synthetic efforts toward the chlorinated aza-propellane alkaloid acutumine (1) are described. The key vicinal quaternary centers were constructed by a photochemical [2 + 2] cycloaddition reaction of a furanyl-tetrahydroindolone. Dihydroxylation of the [2 + 2] product enabled a tandem retro-aldol/intramolecular ketalization reaction, which revealed the aza-propellane core of 1 while generating an unusual, caged, pentacyclic hemiketal product.
Figures

Similar articles
-
Total synthesis of the congested propellane alkaloid (-)-acutumine.Chem Rec. 2014 Aug;14(4):580-91. doi: 10.1002/tcr.201400005. Epub 2014 May 23. Chem Rec. 2014. PMID: 24863243
-
Total syntheses of (-)-acutumine and (-)-dechloroacutumine.Angew Chem Int Ed Engl. 2013 Mar 25;52(13):3642-5. doi: 10.1002/anie.201210076. Epub 2013 Feb 20. Angew Chem Int Ed Engl. 2013. PMID: 23427090 No abstract available.
-
Synthesis of the core structure of acutumine.Org Lett. 2005 Mar 17;7(6):1089-92. doi: 10.1021/ol050020b. Org Lett. 2005. PMID: 15760146
-
Synthetic chemistry of halichlorine and the pinnaic acids.Chem Rev. 2005 Dec;105(12):4483-514. doi: 10.1021/cr0406209. Chem Rev. 2005. PMID: 16351051 Review. No abstract available.
-
Total synthesis and biosynthesis of the paraherquamides: an intriguing story of the biological Diels-Alder construction.Chem Pharm Bull (Tokyo). 2002 Jun;50(6):711-40. doi: 10.1248/cpb.50.711. Chem Pharm Bull (Tokyo). 2002. PMID: 12045324 Review.
Cited by
-
Synthetic Strategies toward Natural Products Containing Contiguous Stereogenic Quaternary Carbon Atoms.Angew Chem Int Ed Engl. 2016 Mar 18;55(13):4156-86. doi: 10.1002/anie.201507549. Epub 2016 Feb 2. Angew Chem Int Ed Engl. 2016. PMID: 26836448 Free PMC article. Review.
-
Unified divergent strategy towards the total synthesis of the three sub-classes of hasubanan alkaloids.Nat Commun. 2021 Jan 4;12(1):36. doi: 10.1038/s41467-020-20274-1. Nat Commun. 2021. PMID: 33397993 Free PMC article.
-
Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions.Chem Rev. 2016 Sep 14;116(17):9748-815. doi: 10.1021/acs.chemrev.5b00723. Epub 2016 Mar 28. Chem Rev. 2016. PMID: 27018601 Free PMC article.
-
A synthesis of oxo-thioxo[3.3.3]propellanes from dithiocarbamates and ninhydrin-malononitrile adduct.Mol Divers. 2017 Nov;21(4):849-854. doi: 10.1007/s11030-017-9761-8. Epub 2017 Jun 26. Mol Divers. 2017. PMID: 28653127
References
-
-
Original isolation paper: Goto K, Sudzuki H. Bull Chem Soc Jpn. 1929;4:220.
-
-
-
Structural assignment: Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Chem Pharm Bull. 1971;19:770.Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Tetrahedron Lett. 1967:2421.Tomita M, Okamoto Y, Kikuchi T, Osaki K, Nishikawa M, Kamiya K, Sasaki Y, Matoba K, Goto K. Tetrahedron Lett. 1967:2425.
-
-
- Yu BW, Chen JY, Wang YP, Cheng KF, Li XY, Qin GW. Phytochemistry. 2002;61:439. - PubMed
-
- Qin GW, Tang XC, Lestage P, Caignard DH, Renard P. PCT Int Appl WO 2004000815. 2003
-
-
For synthetic studies toward acutumine, see: Nguyen TX. PhD Thesis. University of California; San Diego: 2009. Moreau RJ, Sorensen EJ. Tetrahedron. 2007;63:6446.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

