Membrane organization and dynamics of "inner pair" and "outer pair" tryptophan residues in gramicidin channels
- PMID: 22892073
- PMCID: PMC3442126
- DOI: 10.1021/jp304846f
Membrane organization and dynamics of "inner pair" and "outer pair" tryptophan residues in gramicidin channels
Abstract
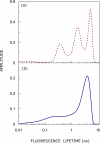
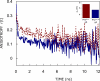
The linear ion channel peptide gramicidin serves as an excellent prototype for monitoring the organization, dynamics, and function of membrane-spanning channels. The tryptophan residues in gramicidin channels are crucial for establishing and maintaining the structure and function of the channel in the membrane bilayer. In order to address the basis of differential importance of tryptophan residues in the gramicidin channel, we monitored the effects of pairwise substitution of two of the four gramicidin tryptophans, the inner pair (Trp-9 and -11) and the outer pair (Trp-13 and -15), using a combination of steady state and time-resolved fluorescence approaches and circular dichroism spectroscopy. We show here that these double tryptophan gramicidin analogues adopt different conformations in membranes, suggesting that the conformational preference of double tryptophan gramicidin analogues is dictated by the positions of the tryptophans in the sequence. These results assume significance in the context of recent observations that the inner pair of tryptophans (Trp-9 and -11) is more important for gramicidin channel formation and channel conductance. These results could be potentially useful in analyzing the effect of tryptophan substitution on the functioning of ion channels and membrane proteins.
Figures



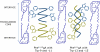
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

