Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome
- PMID: 22892107
- PMCID: PMC3476237
- DOI: 10.1189/jlb.0112014
Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome
Abstract
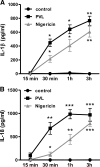
The Staphylococcus aureus pore-forming toxin PVL is most likely causative for life-threatening necrotizing infections, which are characterized by massive tissue inflammation and necrosis. Whereas the cytotoxic action of PVL on human neutrophils is already well established, the PVL effects on other sensitive cell types, such as monocytes and macrophages, are less clear. In this study, we used different types of human leukocytes (neutrophils, monocytes, macrophages, lymphocytes) to investigate cell-specific binding of PVL subunits and subsequent proinflammatory and cytotoxic effects. In all PVL-sensitive cells, we identified the binding of the subunit LukS-PV as the critical factor for PVL-induced cytotoxicity, which was followed by binding of LukF-PV. LukS-PV binds to monocytes, macrophages, and neutrophils but not to lymphocytes. Additionally, we showed that PVL binding to monocytes and macrophages leads to release of caspase-1-dependent proinflammatory cytokines IL-1β and IL-18. PVL activates the NLRP3 inflammasome, a signaling complex of myeloid cells that is involved in caspase-1-dependent IL-1β processing in response to pathogens and endogenous danger signals. Specific inhibition of this pathway at several steps significantly reduced inflammasome activation and subsequent pyronecrosis. Furthermore, we found that PAMPs and DAMPs derived from dying neutrophils can dramatically enhance this response by up-regulating pro-IL-1β in monocytes/macrophages. This study analyzes a specific host signaling pathway that mediates PVL-induced inflammation and cytotoxicity, which has high relevance for CA-MRSA-associated and PVL-mediated pathogenic processes, such as necrotizing infections.
Figures







References
-
- Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 - PubMed
-
- Kaneko J., Kamio Y. (2004) Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 68, 981–1003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

