Regulation of the pro-inflammatory cytokine osteopontin by GIP in adipocytes--a role for the transcription factor NFAT and phosphodiesterase 3B
- PMID: 22892131
- PMCID: PMC3759516
- DOI: 10.1016/j.bbrc.2012.07.157
Regulation of the pro-inflammatory cytokine osteopontin by GIP in adipocytes--a role for the transcription factor NFAT and phosphodiesterase 3B
Abstract
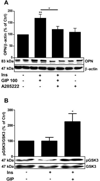
The incretin - glucose-dependent insulinotropic polypeptide (GIP) - and the pro-inflammatory cytokine osteopontin are known to have important roles in the regulation of adipose tissue functions. In this work we show that GIP stimulates lipogenesis and osteopontin expression in primary adipocytes. The GIP-induced increase in osteopontin expression was inhibited by the NFAT (the transcription factor nuclear factor of activated T-cells) inhibitor A-285222. Also, the NFAT kinase glycogen synthase kinase (GSK) 3 was upregulated by GIP. To test whether cAMP might be involved in GIP-mediated effects on osteopontin a number of strategies were used. Thus, the β3-adrenergic receptor agonist CL316,243 stimulated osteopontin expression, an effects which was mimicked by OPC3911, a specific inhibitor of phosphodiesterase 3. Furthermore, treatment of phosphodiesterase 3B knock-out mice with CL316,243 resulted in a dramatic upregulation of osteopontin in adipose tissue which was not the case in wild-type mice. In summary, we delineate mechanisms by which GIP stimulates osteopontin in adipocytes. Given the established link between osteopontin and insulin resistance, our data suggest that GIP by stimulating osteopontin expression, also could promote insulin resistance in adipocytes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

