Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats
- PMID: 22892341
- PMCID: PMC3477211
- DOI: 10.1124/jpet.112.197780
Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats
Abstract
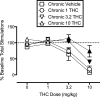
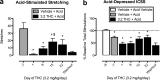
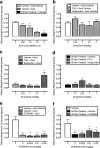
Cannabinoid receptor agonists produce reliable antinociception in most preclinical pain assays but have inconsistent analgesic efficacy in humans. This disparity suggests that conventional preclinical assays of nociception are not sufficient for the prediction of cannabinoid effects related to clinical analgesia. To extend the range of preclinical cannabinoid assessment, this study compared the effects of the marijuana constituent and low-efficacy cannabinoid agonist Δ9-tetrahydrocannabinol (THC) and the high-efficacy synthetic cannabinoid agonist 3-(2-hydroxy-4-(1,1-dimethylheptyl)phenyl)-4-(3-hydroxypropyl)cyclohexanol (CP55940) in assays of pain-stimulated and pain-depressed behavior. Intraperitoneal injection of dilute lactic acid (1.8% in 1 ml/kg) stimulated a stretching response or depressed intracranial self-stimulation (ICSS) in separate groups of male Sprague-Dawley rats. THC (0.1-10 mg/kg) and CP55940 (0.0032-0.32 mg/kg) dose-dependently blocked acid- stimulated stretching but only exacerbated acid-induced depression of ICSS at doses that also decreased control ICSS in the absence of a noxious stimulus. Repeated THC produced tolerance to sedative rate-decreasing effects of THC on control ICSS in the absence of the noxious stimulus but failed to unmask antinociception in the presence of the noxious stimulus. THC and CP55940 also failed to block pain-related depression of feeding in rats, although THC did attenuate satiation-related depression of feeding. In contrast to the effects of the cannabinoid agonists, the clinically effective analgesic and nonsteroidal anti-inflammatory drug ketoprofen (1 mg/kg) blocked acid-stimulated stretching and acid-induced depression of both ICSS and feeding. The poor efficacy of THC and CP55940 to block acute pain-related depression of behavior in rats agrees with the poor efficacy of cannabinoids to treat acute pain in humans.
Figures





References
-
- Anikwue R, Huffman JW, Martin ZL, Welch SP. (2002) Decrease in efficacy and potency of nonsteroidal anti-inflammatory drugs by chronic Δ9-tetrahydrocannabinol administration. J Pharmacol Exp Ther 303:340–346 - PubMed
-
- Beaulieu P. (2006) Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth 53:769–775 - PubMed
-
- Blackburn-Munro G. (2004) Pain-like behaviours in animals–how human are they? Trends Pharmacol Sci 25:299–305 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

