Predictive value of HER2 serum levels in patients treated with lapatinib or trastuzumab -- a translational project in the neoadjuvant GeparQuinto trial
- PMID: 22892393
- PMCID: PMC3464767
- DOI: 10.1038/bjc.2012.353
Predictive value of HER2 serum levels in patients treated with lapatinib or trastuzumab -- a translational project in the neoadjuvant GeparQuinto trial
Abstract
Background: We were able to demonstrate a predictive value of serum HER2 (sHER2) in patients receiving trastuzumab in the neoadjuvant GeparQuattro trial. However, the role of sHER2 in patients receiving neoadjuvant therapy (NT) with lapatinib is still unclear.
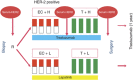
Methods: The neoadjuvant GeparQuinto trial compared trastuzumab vs lapatinib in addition to chemotherapy in HER2-positive primary breast cancer patients. The sHER2 levels were measured by enzyme-linked immunosorbant assay in 210 patients, of whom 109 (52%) patients received trastuzumab and 101 (48%) lapatinib at three different time points.
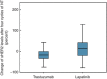
Results: Twenty-two percent of patients had elevated baseline sHER2 levels (>15 ng ml⁻¹). A decrease of sHER2 levels (>20%) in the trastuzumab and lapatinib-treated group during NT was seen in 44% and 24% of the patients, an increase of sHER2 levels (>20%) was seen in 6% and 41% of patients, respectively. Higher pre-chemotherapy sHER2 levels were associated with higher pathological complete remission (pCR) rates in the entire study cohort (OR 1.8, 95% CI 1.02-3.2, P=0.043). A decline of sHER2 levels (>20%) during NT was a predictor for pCR in the lapatinib-treated patient group (OR: 11.7, 95% CI 1.3-110, P=0.031).
Conclusion: Results of this study demonstrate that sHER2 levels change differently during NT depending on the anti-HER2 treatment strategy. Elevated baseline sHER2 levels (>15 ng ml⁻¹) and a decrease of sHER2 levels (>20%) early after therapy initiation are both relevant criteria to predict response to lapatinib-based treatment.
© 2012 Cancer Research UK
Conflict of interest statement
Advisory role/remuneration: Gunter von Minckwitz (Roche), Holger Eidtmann (Roche), Tanja Fehm (Roche), Joachim Bischoff (Roche), Peter Fasching (Novartis), Brigitte Rack (Roche, Novartis and GlaxoSmithKline), Jens Huober (Roche and GlaxoSmithKline) and Volkmar Müller (Amgen, Celgene, Sanofi-Aventis, Pierre-Fabre and Roche). Honoraria: Gunter von Minckwitz (Roche) and Peter Fasching (Novartis). Research funding: Gunter von Minckwitz (GlaxoSmithKline and Roche), Tanja Fehm (Roche, Novartis and GlaxoSmithKline), Joachim Bischoff (GlaxoSmithKline), Peter Fasching (Novartis), Jens Huober (GlaxoSmithKline) and Volkmar Müller (Roche). The remaining authors declare no conflict of interest.
Figures
References
-
- Ali SM, Carney WP, Esteva FJ, Fornier M, Harris L, Kostler WJ, Lotz JP, Luftner D, Pichon MF, Lipton A (2008) Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer 113: 1294–1301 - PubMed
-
- Baselga J (2001) Is circulating HER-2 more than just a tumor marker? Clin Cancer Res 7: 2605–2607 - PubMed
-
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horvath Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379: 596–598 - PMC - PubMed
-
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23: 3676–3685 - PubMed
-
- Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J (1999) Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res 59: 1196–1201 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

