Induction of antitumor immunity ex vivo using dendritic cells transduced with fowl pox vector expressing MUC1, CEA, and a triad of costimulatory molecules (rF-PANVAC)
- PMID: 22892452
- PMCID: PMC3472644
- DOI: 10.1097/CJI.0b013e31826a73de
Induction of antitumor immunity ex vivo using dendritic cells transduced with fowl pox vector expressing MUC1, CEA, and a triad of costimulatory molecules (rF-PANVAC)
Abstract
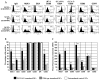
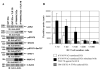
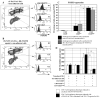
The fowl pox vector expressing the tumor-associated antigens, mucin-1 and carcinoembryonic antigen in the context of costimulatory molecules (rF-PANVAC) has shown promise as a tumor vaccine. However, vaccine-mediated expansion of suppressor T-cell populations may blunt clinical efficacy. We characterized the cellular immune response induced by ex vivo dendritic cells (DCs) transduced with (rF)-PANVAC. Consistent with the functional characteristics of potent antigen-presenting cells, rF-PANVAC-DCs demonstrated strong expression of mucin-1 and carcinoembryonic antigen and costimulatory molecules, CD80, CD86, and CD83; decreased levels of phosphorylated STAT3, and increased levels of Tyk2, Janus kinase 2, and STAT1. rF-PANVAC-DCs stimulated expansion of tumor antigen-specific T cells with potent cytolytic capacity. However, rF-PANVAC-transduced DCs also induced the concurrent expansion of FOXP3 expressing CD4CD25 regulatory T cells (Tregs) that inhibited T-cell activation. Moreover, Tregs expressed high levels of Th2 cytokines [interleukin (IL)-10, IL-4, IL-5, and IL-13] together with phosphorylated STAT3 and STAT6. In contrast, the vaccine-expanded Treg population expressed high levels of Th1 cytokines IL-2 and interferon-γ and the proinflammatory receptor-related orphan receptor γt (RORγt) and IL-17A suggesting that these cells may share effector functions with conventional TH17 T cells. These data suggest that Tregs expanded by rF-PANVAC-DCs, exhibit immunosuppressive properties potentially mediated by Th2 cytokines, but simultaneous expression of Th1 and Th17-associated factors suggests a high degree of plasticity.
Figures


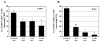

References
-
- Tsang KY, Palena C, Yokokawa J, et al. Analyses of recombinant vaccinia and fowlpox vaccine vectors expressing transgenes for two human tumor antigens and three human costimulatory molecules. Clin Cancer Res. 2005;11:1597–1607. - PubMed
-
- Kass E, Schlom J, Thompson J, et al. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59:676–83. - PubMed
-
- Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

