Cacnb4 directly couples electrical activity to gene expression, a process defective in juvenile epilepsy
- PMID: 22892567
- PMCID: PMC3442274
- DOI: 10.1038/emboj.2012.226
Cacnb4 directly couples electrical activity to gene expression, a process defective in juvenile epilepsy
Abstract
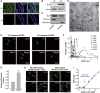
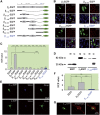
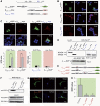
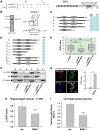
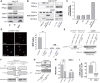
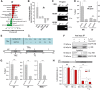
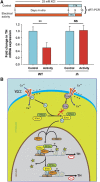
Calcium current through voltage-gated calcium channels (VGCC) controls gene expression. Here, we describe a novel signalling pathway in which the VGCC Cacnb4 subunit directly couples neuronal excitability to transcription. Electrical activity induces Cacnb4 association to Ppp2r5d, a regulatory subunit of PP2A phosphatase, followed by (i) nuclear translocation of Cacnb4/Ppp2r5d/PP2A, (ii) association with the tyrosine hydroxylase (TH) gene promoter through the nuclear transcription factor thyroid hormone receptor alpha (TRα), and (iii) histone binding through association of Cacnb4 with HP1γ concomitantly with Ser(10) histone H3 dephosphorylation by PP2A. This signalling cascade leads to TH gene repression by Cacnb4 and is controlled by the state of interaction between the SH3 and guanylate kinase (GK) modules of Cacnb4. The human R482X CACNB4 mutation, responsible for a form of juvenile myoclonic epilepsy, prevents association with Ppp2r5 and nuclear targeting of the complex by altering Cacnb4 conformation. These findings demonstrate that an intact VGCC subunit acts as a repressor recruiting platform to control neuronal gene expression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Arikkath J, Campbell KP (2003) Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 13: 298–307 - PubMed
-
- Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Ikeda H, Yamada Y, Seino Y, Hunziker W (2006) Nuclear sequestration of beta-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J Mol Biol 355: 34–46 - PubMed
-
- Bengzon J, Hansson SR, Hoffman BJ, Lindvall O (1999) Regulation of norepinephrine transporter and tyrosine hydroxylase mRNAs after kainic acid-induced seizures. Brain Res 842: 239–242 - PubMed
-
- Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M (2000) The I-II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron 25: 177–190 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

