Cancer cell spheroids as a model to evaluate chemotherapy protocols
- PMID: 22892843
- PMCID: PMC3469478
- DOI: 10.4161/cbt.21353
Cancer cell spheroids as a model to evaluate chemotherapy protocols
Abstract
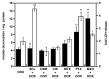
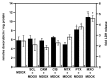
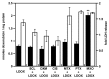
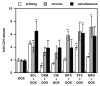
To determine whether the spheroid culture can be used to evaluate drug efficacy, we have evaluated the toxicity of free or carrier-associated doxorubicin as a single drug or in combination with other antineoplastic agents using the spheroid cultures of drug-resistant cancer cells. Paclitaxel, cisplatin, dexamethasone, mitoxantrone, sclareol or methotrexate were used in combination with doxorubicin. The effect of the treatment protocols on free, micellar and liposomal doxorubicin accumulation in spheroids and on resulting toxicity was evaluated by fluorescence and lactate dehydrogenase release, respectively. Enhanced doxorubicin accumulation and toxicity were observed after spheroid pretreatment with mitoxantrone or paclitaxel. Effects of the drug combination with doxorubicin were sequence dependent, use of doxorubicin as the first drug being the least inducer of toxicity. Finally, spheroids were recognized by a cancer cell-specific antibody. Our results suggest the usefulness of spheroids to evaluate chemotherapy combinations.
Figures



References
-
- World Cancer Report, Boyle P and Levin B, Editors. 2008, International Agency for Research on Cancer: Lyon.
-
- Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;8:878–84. - PubMed
-
- Lankelma J, Dekker H, Luque FR, Luykx S, Hoekman K, van der Valk P, et al. Doxorubicin gradients in human breast cancer. Clin Cancer Res. 1999;5:1703–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
