FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage
- PMID: 22893124
- PMCID: PMC3589124
- DOI: 10.1038/ncomms2008
FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage
Erratum in
- Nat Commun. 2013;4:1520
Abstract
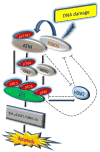
DNA damage as a result of environmental stress is recognized by sensor proteins that trigger repair mechanisms, or, if repair is unsuccessful, initiate apoptosis. Defects in DNA damage-induced apoptosis promote genomic instability and tumourigenesis. The protein ataxia-telangiectasia mutated (ATM) is activated by DNA double-strand breaks and regulates apoptosis via p53. Here we show that FOXO3 interacts with the ATM-Chk2-p53 complex, augments phosphorylation of the complex and induces the formation of nuclear foci in cells on DNA damage. FOXO3 is essential for DNA damage-induced apoptosis and conversely FOXO3 requires ATM, Chk2 and phosphorylated p53 isoforms to trigger apoptosis as a result of DNA damage. Under these conditions FOXO3 may also have a role in regulating chromatin retention of phosphorylated p53. These results suggest an essential link between FOXO3 and the ATM-Chk2-p53-mediated apoptotic programme following DNA damage.
Conflict of interest statement
Figures



Similar articles
-
ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis.J Biol Chem. 2008 Mar 7;283(10):6572-83. doi: 10.1074/jbc.M707568200. Epub 2007 Dec 27. J Biol Chem. 2008. PMID: 18162465
-
STAT-1 facilitates the ATM activated checkpoint pathway following DNA damage.J Cell Sci. 2005 Apr 15;118(Pt 8):1629-39. doi: 10.1242/jcs.01728. Epub 2005 Mar 22. J Cell Sci. 2005. Retraction in: J Cell Sci. 2015 Mar 1;128(5):1064. doi: 10.1242/jcs.169243. PMID: 15784679 Retracted.
-
Functional interaction between FOXO3a and ATM regulates DNA damage response.Nat Cell Biol. 2008 Apr;10(4):460-7. doi: 10.1038/ncb1709. Epub 2008 Mar 16. Nat Cell Biol. 2008. PMID: 18344987 Free PMC article.
-
The ATM protein kinase: regulating the cellular response to genotoxic stress, and more.Nat Rev Mol Cell Biol. 2013 Apr;14(4):197-210. doi: 10.1038/nrm3546. Epub 2013 Mar 13. Nat Rev Mol Cell Biol. 2013. PMID: 23486281 Review.
-
The ATM protein kinase and cellular redox signaling: beyond the DNA damage response.Trends Biochem Sci. 2012 Jan;37(1):15-22. doi: 10.1016/j.tibs.2011.10.002. Epub 2011 Nov 11. Trends Biochem Sci. 2012. PMID: 22079189 Free PMC article. Review.
Cited by
-
miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway.Oncotarget. 2015 Apr 10;6(10):8286-99. doi: 10.18632/oncotarget.3221. Oncotarget. 2015. PMID: 25823655 Free PMC article.
-
Organelle-specific initiation of cell death.Nat Cell Biol. 2014 Aug;16(8):728-36. doi: 10.1038/ncb3005. Nat Cell Biol. 2014. PMID: 25082195 Review.
-
Elucidating the genotoxicity of Fusobacterium nucleatum-secreted mutagens in colorectal cancer carcinogenesis.Gut Pathog. 2024 Sep 27;16(1):50. doi: 10.1186/s13099-024-00640-w. Gut Pathog. 2024. PMID: 39334474 Free PMC article.
-
Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells.Mol Med Rep. 2016 Oct;14(4):3777-84. doi: 10.3892/mmr.2016.5706. Epub 2016 Sep 5. Mol Med Rep. 2016. PMID: 27599894 Free PMC article.
-
Auranofin displays anticancer activity against ovarian cancer cells through FOXO3 activation independent of p53.Int J Oncol. 2014 Oct;45(4):1691-8. doi: 10.3892/ijo.2014.2579. Epub 2014 Aug 4. Int J Oncol. 2014. PMID: 25096914 Free PMC article.
References
-
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. - PubMed
-
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. - PubMed
-
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. - PubMed
-
- Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. - PubMed
-
- Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

