Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease
- PMID: 22893382
- PMCID: PMC3419519
- DOI: 10.1128/mBio.00180-12
Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease
Abstract
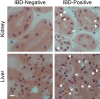
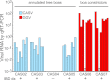
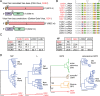
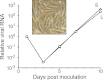
Inclusion body disease (IBD) is an infectious fatal disease of snakes typified by behavioral abnormalities, wasting, and secondary infections. At a histopathological level, the disease is identified by the presence of large eosinophilic cytoplasmic inclusions in multiple tissues. To date, no virus or other pathogen has been definitively characterized or associated with the disease. Using a metagenomic approach to search for candidate etiologic agents in snakes with confirmed IBD, we identified and de novo assembled the complete genomic sequences of two viruses related to arenaviruses, and a third arenavirus-like sequence was discovered by screening an additional set of samples. A continuous boa constrictor cell line was established and used to propagate and isolate one of the viruses in culture. Viral nucleoprotein was localized and concentrated within large cytoplasmic inclusions in infected cells in culture and tissues from diseased snakes. In total, viral RNA was detected in 6/8 confirmed IBD cases and 0/18 controls. These viruses have a typical arenavirus genome organization but are highly divergent, belonging to a lineage separate from that of the Old and New World arenaviruses. Furthermore, these viruses encode envelope glycoproteins that are more similar to those of filoviruses than to those of other arenaviruses. These findings implicate these viruses as candidate etiologic agents of IBD. The presence of arenaviruses outside mammals reveals that these viruses infect an unexpectedly broad range of species and represent a new reservoir of potential human pathogens.
Importance: Inclusion body disease (IBD) is a common infectious disease of captive snakes. IBD is fatal and can cause the loss of entire animal collections. The cause of the disease has remained elusive, and no treatment exists. In addition to being important to pet owners, veterinarians, breeders, zoological parks, and aquariums, the study of animal disease is significant since animals are the source of virtually every emerging infectious human disease. We searched for candidate causative agents in snakes diagnosed with IBD and found a group of novel viruses distantly related mainly to arenaviruses but also to filoviruses, both of which can cause fatal hemorrhagic fevers when transmitted from animals to humans. In addition to providing evidence that strongly suggests that these viruses cause snake IBD, this discovery reveals a new and unanticipated domain of virus biology and evolution.
Figures

References
-
- Chang L-W, Jacobson ER. 2010. Inclusion body disease, a worldwide infectious disease of boid snakes: a review. J. Exot. Pet Med. 19:216–225
-
- Axthelm M. 1985. Clinicopathologic and virologic observations of a probable viral disease affecting boid snakes. Proc. Annu. Meet. Am. Assoc. Zoo Vet. 1985:108
-
- Schumacher J, Jacobson E, Homer B, Gaskin J. 1994. Inclusion body disease in boid snakes. J. Zoo Wildl. Med. 25:511–524
-
- Jacobson ER, et al. 2001. Partial characterization of retroviruses from boid snakes with inclusion body disease. Am. J. Vet. Res. 62:217–224 - PubMed
-
- Wozniak E, et al. 2000. Isolation and characterization of an antigenically distinct 68-kd protein from nonviral intracytoplasmic inclusions in boa constrictors chronically infected with the inclusion body disease virus (IBDV: Retroviridae). Vet. Pathol. 37:449–459 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

