Early cardiac development: a view from stem cells to embryos
- PMID: 22893679
- PMCID: PMC3500045
- DOI: 10.1093/cvr/cvs270
Early cardiac development: a view from stem cells to embryos
Abstract
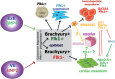
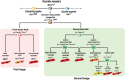
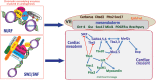
From the 1920s, early cardiac development has been studied in chick and, later, in mouse embryos in order to understand the first cell fate decisions that drive specification and determination of the endocardium, myocardium, and epicardium. More recently, mouse and human embryonic stem cells (ESCs) have demonstrated faithful recapitulation of early cardiogenesis and have contributed significantly to this research over the past few decades. Derived almost 15 years ago, human ESCs have provided a unique developmental model for understanding the genetic and epigenetic regulation of early human cardiogenesis. Here, we review the biological concepts underlying cell fate decisions during early cardiogenesis in model organisms and ESCs. We draw upon both pioneering and recent studies and highlight the continued role for in vitro stem cells in cardiac developmental biology.
Figures

References
-
- van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011;8:50–60. doi:10.1038/nrcardio.2010.166. - DOI - PubMed
-
- Tararbit K, Houyel L, Bonnet D, De Vigan C, Lelong N, Goffinet F, et al. Risk of congenital heart defects associated with assisted reproductive technologies: a population-based evaluation. Eur Heart J. 2011;32:500–508. doi:10.1093/eurheartj/ehq440. - DOI - PubMed
-
- Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci USA. 1975;72:1441–1445. doi:10.1073/pnas.72.4.1441. - DOI - PMC - PubMed
-
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi:10.1073/pnas.78.12.7634. - DOI - PMC - PubMed
-
- Capecchi MR. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989;5:70–76. doi:10.1016/0168-9525(89)90029-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

