Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients
- PMID: 22893706
- PMCID: PMC3471767
- DOI: 10.1074/jbc.M112.367532
Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients
Abstract
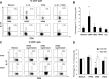
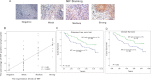
The accumulation of an intratumoral CD4(+) interleukin-17-producing subset (Th17) of tumor-infiltrating lymphocytes (TILs) is a general characteristic in many cancers. The relationship between the percentage of Th17 cells and clinical prognosis differs among cancers. The mechanism responsible for the increasing percentage of such cells in NPC is still unknown, as is their biological function. Here, our data showed an increase of Th17 cells in tumor tissues relative to their numbers in normal nasopharynx tissues or in the matched peripheral blood of NPC patients. Th17 cells in tumor tissue produced more IFNγ than did those in the peripheral blood of matched NPC patients and healthy controls. We observed high levels of CD154, G-CSF, CXCL1, IL-6, IL-8, and macrophage inhibitory factor (MIF) out of 36 cytokines examined in tumor tissue cultures. MIF promoted the generation and recruitment of Th17 cells mediated by NPC tumor cells in vitro; this promoting effect was mainly dependent on the mammalian target of rapamycin pathway and was mediated by the MIF-CXCR4 axis. Finally, the expression level of MIF in tumor cells and in TILs was positively correlated in NPC tumor tissues, and the frequency of MIF-positive TILs was positively correlated with NPC patient clinical outcomes. Taken together, our findings illustrate that tumor-derived MIF can affect patient prognosis, which might be related to the increase of Th17 cells in the NPC tumor microenvironment.
Figures





Similar articles
-
Increased expression of macrophage migration inhibitory factor and DJ-1 contribute to cell invasion and metastasis of nasopharyngeal carcinoma.Int J Med Sci. 2013 Dec 22;11(1):106-15. doi: 10.7150/ijms.7264. eCollection 2014. Int J Med Sci. 2013. PMID: 24396292 Free PMC article.
-
The expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinoma.J Transl Med. 2013 Mar 8;11:60. doi: 10.1186/1479-5876-11-60. J Transl Med. 2013. PMID: 23497377 Free PMC article.
-
MIF promotes a differential Th1/Th2/Th17 inflammatory response in human primary cell cultures: Predominance of Th17 cytokine profile in PBMC from healthy subjects and increase of IL-6 and TNF-α in PBMC from active SLE patients.Cell Immunol. 2018 Feb;324:42-49. doi: 10.1016/j.cellimm.2017.12.010. Epub 2017 Dec 26. Cell Immunol. 2018. PMID: 29397904
-
Macrophage migration inhibitory factor involvement in breast cancer (Review).Int J Oncol. 2015 Nov;47(5):1627-33. doi: 10.3892/ijo.2015.3185. Epub 2015 Sep 24. Int J Oncol. 2015. PMID: 26412712 Free PMC article. Review.
-
Expression of chemokine receptor CXCR4 is closely correlated with clinical outcome in human nasopharyngeal carcinoma.Tumour Biol. 2016 May;37(5):6099-105. doi: 10.1007/s13277-015-4464-1. Epub 2015 Nov 26. Tumour Biol. 2016. PMID: 26611644
Cited by
-
Identification of DTL as Related Biomarker and Immune Infiltration Characteristics of Nasopharyngeal Carcinoma via Comprehensive Strategies.Int J Gen Med. 2022 Mar 2;15:2329-2345. doi: 10.2147/IJGM.S352330. eCollection 2022. Int J Gen Med. 2022. PMID: 35264872 Free PMC article.
-
TA-MSCs, TA-MSCs-EVs, MIF: their crosstalk in immunosuppressive tumor microenvironment.J Transl Med. 2022 Jul 16;20(1):320. doi: 10.1186/s12967-022-03528-y. J Transl Med. 2022. PMID: 35842634 Free PMC article. Review.
-
IL-17-Producing Cells in Tumor Immunity: Friends or Foes?Immune Netw. 2020 Feb 7;20(1):e6. doi: 10.4110/in.2020.20.e6. eCollection 2020 Feb. Immune Netw. 2020. PMID: 32158594 Free PMC article. Review.
-
Increased expression of IRF8 in tumor cells inhibits the generation of Th17 cells and predicts unfavorable survival of diffuse large B cell lymphoma patients.Oncotarget. 2017 Jul 25;8(30):49757-49772. doi: 10.18632/oncotarget.17693. Oncotarget. 2017. PMID: 28537908 Free PMC article.
-
Macrophage migration inhibitory factor interacting with Th17 cells may be involved in the pathogenesis of autoimmune damage in Hashimoto's thyroiditis.Mediators Inflamm. 2015;2015:621072. doi: 10.1155/2015/621072. Epub 2015 Mar 15. Mediators Inflamm. 2015. PMID: 25861163 Free PMC article.
References
-
- Wee J. T., Ha T. C., Loong S. L., Qian C. N. (2010) Is nasopharyngeal cancer really a “Cantonese cancer”? Chin. J. Cancer 29, 517–526 - PubMed
-
- Niedobitek G., Agathanggelou A., Nicholls J. M. (1996) Epstein-Barr virus infection and the pathogenesis of nasopharyngeal carcinoma. Viral gene expression, tumor cell phenotype, and the role of the lymphoid stroma. Semin. Cancer Biol. 7, 165–174 - PubMed
-
- Vasef M. A., Ferlito A., Weiss L. M. (1997) Nasopharyngeal carcinoma, with emphasis on its relationship to Epstein-Barr virus. Ann. Otol. Rhinol. Laryngol. 106, 348–356 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

