Exoribonuclease and endoribonuclease activities of RNase BN/RNase Z both function in vivo
- PMID: 22893707
- PMCID: PMC3471727
- DOI: 10.1074/jbc.M112.407403
Exoribonuclease and endoribonuclease activities of RNase BN/RNase Z both function in vivo
Abstract
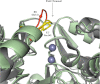
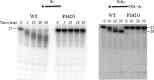
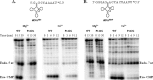
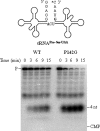
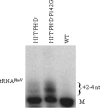
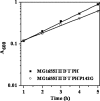
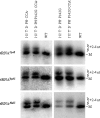
Escherichia coli RNase BN, a member of the RNase Z family of endoribonucleases, differs from other family members in that it also can act as an exoribonuclease in vitro. Here, we examine whether this activity of RNase BN also functions in vivo. Comparison of the x-ray structure of RNase BN with that of Bacillus subtilis RNase Z, which lacks exoribonuclease activity, revealed that RNase BN has a narrower and more rigid channel downstream of the catalytic site. We hypothesized that this difference in the putative RNA exit channel might be responsible for the acquisition of exoribonuclease activity by RNase BN. Accordingly, we generated several mutant RNase BN proteins in which residues within a loop in this channel were converted to the corresponding residues present in B. subtilis RNase Z, thus widening the channel and increasing its flexibility. The resulting mutant RNase BN proteins had reduced or were essentially devoid of exoribonuclease activity in vitro. Substitution of one mutant rbn gene (P142G) for wild type rbn in the E. coli chromosome revealed that the exoribonuclease activity of RNase BN is not required for maturation of phage T4 tRNA precursors, a known specific function of this RNase. On the other hand, removal of the exoribonuclease activity of RNase BN in a cell lacking other processing RNases leads to slower growth and affects maturation of multiple tRNA precursors. These findings help explain how RNase BN can act as both an exo- and an endoribonuclease and also demonstrate that its exoribonuclease activity is capable of functioning in vivo, thus widening the potential role of this enzyme in E. coli.
Figures
References
-
- Deutscher M. P. (1990) Ribonucleases, tRNA nucleotidyltransferase, and the 3′ processing of tRNA. Prog. Nucleic Acids Res. Mol. Biol. 39, 209–240 - PubMed
-
- Deutscher M. P. (1995) tRNA. Structure, Biosynthesis, and Function (Soll D., Rajbhandary U. L., eds) pp. 51–65, American Society for Microbiology, Washington, D. C
-
- Li Z., Deutscher M. P. (1996) Maturation pathways for E. coli tRNA precursors. A random multienzyme process in vivo. Cell 86, 503–512 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

